Ramucirumab after prior sorafenib in patients with advanced hepatocellular carcinoma and elevated alpha-fetoprotein: Japanese subgroup analysis of the REACH-2 trial
- PMID: 32107609
- PMCID: PMC7242235
- DOI: 10.1007/s00535-020-01668-w
Ramucirumab after prior sorafenib in patients with advanced hepatocellular carcinoma and elevated alpha-fetoprotein: Japanese subgroup analysis of the REACH-2 trial
Abstract
Background: The global, randomized, phase 3 REACH-2 study (ClinicalTrials.gov identifier: NCT02435433) found significantly longer overall survival (OS) for second-line ramucirumab versus placebo (hazard ratio [HR]: 0.710, 95% confidence interval [CI] 0.531-0.949, P = 0.0199) in patients with advanced hepatocellular carcinoma (HCC) and alpha-fetoprotein (AFP) ≥ 400 ng/mL. This prespecified subgroup analysis evaluated the efficacy and safety of ramucirumab in the Japanese patients enrolled in the study.
Methods: Patients with advanced HCC and AFP ≥ 400 ng/mL after first-line sorafenib were randomized 2:1 to ramucirumab (8 mg/kg intravenously) or placebo every 2 weeks. Hazard ratios for progression-free survival (PFS) and OS (primary endpoint of the overall study) were estimated using the stratified Cox regression model. We also pooled individual patient data from REACH-2 with data from REACH (NCT01140347) for patients with AFP ≥ 400 ng/mL.
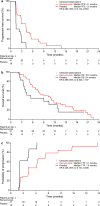
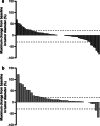
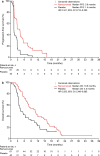
Results: In the Japanese REACH-2 subpopulation, there were improvements for ramucirumab (n = 41) versus placebo (n = 18) in PFS (HR 0.282, 95% CI 0.144-0.553) and OS was numerically prolonged (HR 0.599, 95% CI 0.303-1.187), consistent with the significant benefit seen in the overall REACH-2 study population. In the ramucirumab and placebo arms, respectively, the objective response rate was 7.3% and 0%, and the disease control rate was 70.7% and 33.3%. The most frequently reported grade ≥ 3 treatment-emergent adverse event was hypertension (ramucirumab: 15%; placebo: 11%).
Conclusions: Ramucirumab after prior sorafenib improved PFS and OS compared with placebo, with a manageable safety profile, in the Japanese REACH-2 subpopulation, consistent with the overall REACH-2 study results. Ramucirumab is the first agent to demonstrate clinical benefit for Japanese patients with HCC in the second-line setting.
Keywords: Alpha-fetoprotein; Hepatocellular carcinoma; Japanese subanalysis; Ramucirumab; VEGFR2.
Conflict of interest statement
MK has had a consulting/advisory role with Bayer, Bristol-Myers Squibb, Eisai Co., Ltd., MSD, and Ono Pharmaceutical; has received honoraria from Bayer, EA Pharma Co., Ltd., Eisai Co., Ltd., and MSD; and has received research funding from AbbVie Inc., Astellas Pharma Inc., Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., EA Pharma Co., Ltd., Eisai Co., Ltd., Gilead Sciences, Inc., Medico’s Hirata Inc., Otsuka Pharmaceutical Co., Ltd., Taiho Pharmaceutical, and Takeda Pharmaceutical Company Limited. TO has received research funding from Eli Lilly Japan K.K., AstraZeneca K.K., Baxter, Bristol-Myers Squibb K.K., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Novartis Pharma K.K., and Sumitomo Dainippon Pharma Co., Ltd. KM has received honoraria from Eisai Co., Ltd. TY has received honoraria from Eli Lilly, Bayer, and Eisai Co., Ltd. HT, GH, and RY are employees of Eli Lilly Japan K.K. and own stock in Eli Lilly and Company. IO, MM, SSeo, YW, SSato, MF, TA, SN, KO, HF, TK, JF, and AXZ have no conflicts of interest to declare.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

