Unstructured network topology begets order-based representation by privileged neurons
- PMID: 32107622
- PMCID: PMC7062672
- DOI: 10.1007/s00422-020-00819-9
Unstructured network topology begets order-based representation by privileged neurons
Abstract
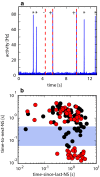
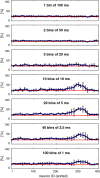
How spiking activity reverberates through neuronal networks, how evoked and spontaneous activity interacts and blends, and how the combined activities represent external stimulation are pivotal questions in neuroscience. We simulated minimal models of unstructured spiking networks in silico, asking whether and how gentle external stimulation might be subsequently reflected in spontaneous activity fluctuations. Consistent with earlier findings in silico and in vitro, we observe a privileged subpopulation of 'pioneer neurons' that, by their firing order, reliably encode previous external stimulation. We also confirm that pioneer neurons are 'sensitive' in that they are recruited by small fluctuations of population activity. We show that order-based representations rely on a 'chain' of pioneer neurons with different degrees of sensitivity and thus constitute an emergent property of collective dynamics. The forming of such representations is greatly favoured by a broadly heterogeneous connection topology-a broad 'middle class' in degree of connectedness. In conclusion, we offer a minimal model for the representational role of pioneer neurons, as observed experimentally in vitro. In addition, we show that broadly heterogeneous connectivity enhances the representational capacity of unstructured networks.
Keywords: Heterogeneous random connectivity; Leader neurons; Motifs; Neural code; Neural dynamics; Neural representation; Pioneer neurons; Spiking networks; Synchronization events.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures









References
-
- Ansmann G, Karnatak R, Lehnertz K, Feudel U. Extreme events in excitable systems and mechanisms of their generation. Phys Rev E. 2013;88(5):052911. - PubMed
-
- Bak P, Tang C, Wiesenfeld K. Self-organized criticality. Phys Rev A. 1988;38(1):364. - PubMed
-
- Barabási AL, Albert R. Emergence of scaling in random networks. Science. 1999;286(5439):509–512. - PubMed
-
- Bauermeister C, Keren H, Braun J (2015) Coherent coupling of in vitro neuronal slices onto in silico networks. In: 11th Bernstein conference, Heidelberg, Germany
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

