Requirement of the acyl-CoA carrier ACBD6 in myristoylation of proteins: Activation by ligand binding and protein interaction
- PMID: 32108178
- PMCID: PMC7046191
- DOI: 10.1371/journal.pone.0229718
Requirement of the acyl-CoA carrier ACBD6 in myristoylation of proteins: Activation by ligand binding and protein interaction
Erratum in
-
Correction: Requirement of the acyl-CoA carrier ACBD6 in myristoylation of proteins: Activation by ligand binding and protein interaction.PLoS One. 2022 Apr 21;17(4):e0267678. doi: 10.1371/journal.pone.0267678. eCollection 2022. PLoS One. 2022. PMID: 35446914 Free PMC article.
Abstract
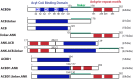
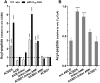
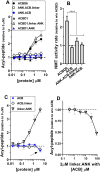
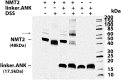
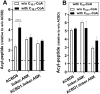
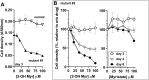
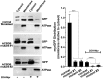
Glycine N-myristoylation is an essential acylation modification modulating the functions, stability, and membrane association of diverse cytosolic proteins in human cells. Myristoyl-CoA is the 14-carbon acyl donor of the acyltransferase reaction. Acyl-CoAs of a chain length compatible with the binding site of the N-myristoyltransferase enzymes (NMT) are competitive inhibitors, and the mechanism protecting these enzymes from unwanted acyl-CoA species requires the acyl-CoA binding protein ACBD6. The acyl-CoA binding domain (ACB) and the ankyrin-repeat motifs (ANK) of ACBD6 can perform their functions independently. Interaction of ANK with human NMT2 was necessary and sufficient to provide protection. Fusion of the ANK module to the acyl-CoA binding protein ACBD1 was sufficient to confer the NMT-stimulatory property of ACBD6 to the chimera. The ACB domain is dispensable and sequestration of the competitor was not the basis for NMT2 protection. Acyl-CoAs bound to ACB modulate the function of the ANK module and act as positive effector of the allosteric activation of the enzyme. The functional relevance of homozygous mutations in ACBD6 gene, which have not been associated with a disease so far, is presented. Skin-derived fibroblasts of two unrelated individuals with neurodevelopmental disorder and carrying loss of function mutations in the ACBD6 gene were deficient in protein N-myristoylation. These cells were sensitive to substrate analog competing for myristoyl-CoA binding to NMT. These findings account for the requirement of an ANK-containing acyl-CoA binding protein in the cellular mechanism protecting the NMT enzymes and establish that in human cells, ACBD6 supports the N-myristoylation of proteins.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Dual Role of ACBD6 in the Acylation Remodeling of Lipids and Proteins.Biomolecules. 2022 Nov 22;12(12):1726. doi: 10.3390/biom12121726. Biomolecules. 2022. PMID: 36551154 Free PMC article.
-
Association of NMT2 with the acyl-CoA carrier ACBD6 protects the N-myristoyltransferase reaction from palmitoyl-CoA.J Lipid Res. 2016 Feb;57(2):288-98. doi: 10.1194/jlr.M065003. Epub 2015 Nov 30. J Lipid Res. 2016. PMID: 26621918 Free PMC article.
-
ACBD6 protein controls acyl chain availability and specificity of the N-myristoylation modification of proteins.J Lipid Res. 2019 Mar;60(3):624-635. doi: 10.1194/jlr.M091397. Epub 2019 Jan 14. J Lipid Res. 2019. PMID: 30642881 Free PMC article.
-
Myristoylation.Cell Signal. 1997 Jan;9(1):15-35. doi: 10.1016/s0898-6568(96)00100-3. Cell Signal. 1997. PMID: 9067626 Review.
-
Access and utilization of long chain fatty acyl-CoA by zDHHC protein acyltransferases.Curr Opin Struct Biol. 2022 Dec;77:102463. doi: 10.1016/j.sbi.2022.102463. Epub 2022 Sep 29. Curr Opin Struct Biol. 2022. PMID: 36183446 Free PMC article. Review.
Cited by
-
N-Myristoyltransferase as a Glycine and Lysine Myristoyltransferase in Cancer, Immunity, and Infections.ACS Chem Biol. 2020 Jul 17;15(7):1747-1758. doi: 10.1021/acschembio.0c00314. Epub 2020 Jun 10. ACS Chem Biol. 2020. PMID: 32453941 Free PMC article. Review.
-
Correction: Requirement of the acyl-CoA carrier ACBD6 in myristoylation of proteins: Activation by ligand binding and protein interaction.PLoS One. 2022 Apr 21;17(4):e0267678. doi: 10.1371/journal.pone.0267678. eCollection 2022. PLoS One. 2022. PMID: 35446914 Free PMC article.
-
Dual Role of ACBD6 in the Acylation Remodeling of Lipids and Proteins.Biomolecules. 2022 Nov 22;12(12):1726. doi: 10.3390/biom12121726. Biomolecules. 2022. PMID: 36551154 Free PMC article.
-
Bi-allelic ACBD6 variants lead to a neurodevelopmental syndrome with progressive and complex movement disorders.Brain. 2024 Apr 4;147(4):1436-1456. doi: 10.1093/brain/awad380. Brain. 2024. PMID: 37951597 Free PMC article.
-
Deciphering the role of lipid metabolism-related genes in Alzheimer's disease: a machine learning approach integrating Traditional Chinese Medicine.Front Endocrinol (Lausanne). 2024 Oct 23;15:1448119. doi: 10.3389/fendo.2024.1448119. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39507054 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

