Clinical trial analysis of 2019-nCoV therapy registered in China
- PMID: 32108352
- PMCID: PMC7228274
- DOI: 10.1002/jmv.25733
Clinical trial analysis of 2019-nCoV therapy registered in China
Abstract
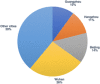
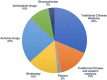
So far, there is a lack of effective drugs for the new coronavirus pneumonia. With more and more patients diagnosed, China has carried out more than 100 clinical studies of new coronavirus infection, including antiviral drugs, antimalarial drugs, glucocorticoids, plasma therapy, virus vaccine, and other Western drugs, while Chinese medicine research accounted for half of the studies. Most of the trials were initiated by investigators and the study period would last for 1 to 11 months. The primary endpoints included symptom improvement and virus nucleic acid turning negative, but the optimal endpoint has not been determined. Although the final results of studies will take a long time to complete, the interim research data may provide some help for the current urgent demand for drug treatment. Compared with that of during SARS period in 2003, China has the stronger capability to carry out clinical trials of new drugs in emergency period.
Keywords: China; clinical trial; new coronavirus pneumonia; new drugs.
© 2020 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures
Comment in
-
Potentially repurposing adamantanes for COVID-19.J Med Virol. 2020 Jun;92(6):531-532. doi: 10.1002/jmv.25752. Epub 2020 Mar 16. J Med Virol. 2020. PMID: 32176361 No abstract available.
-
Comments on Zhang et al: Clinical trial analysis of 2019-nCoV therapy registered in China.J Med Virol. 2020 Jul;92(7):711-712. doi: 10.1002/jmv.25806. Epub 2020 Apr 15. J Med Virol. 2020. PMID: 32239514 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous