New Medical Device Acquisition During Pediatric Severe Sepsis Hospitalizations
- PMID: 32108704
- PMCID: PMC8810235
- DOI: 10.1097/CCM.0000000000004272
New Medical Device Acquisition During Pediatric Severe Sepsis Hospitalizations
Abstract
Objectives: Severe sepsis is a significant cause of healthcare utilization and morbidity among pediatric patients. However, little is known about how commonly survivors acquire new medical devices during pediatric severe sepsis hospitalization. We sought to determine the rate of new device acquisition (specifically, tracheostomy placement, gastrostomy tube placement, vascular access devices, ostomy procedures, and amputation) among children surviving hospitalizations with severe sepsis. For contextualization, we compare this to rates of new device acquisition among three comparison cohorts: 1) survivors of all-cause pediatric hospitalizations; 2) matched survivors of nonsepsis infection hospitalizations; and 3) matched survivors of all-cause nonsepsis hospitalization with similar organ dysfunction.
Design: Observational cohort study.
Setting: Nationwide Readmission Database (2016), including all-payer hospitalizations from 27 states.
Patients: Eighteen-thousand two-hundred ten pediatric severe sepsis hospitalizations; 532,738 all-cause pediatric hospitalizations; 16,173 age- and sex-matched nonsepsis infection hospitalizations; 15,025 organ dysfunction matched all-cause nonsepsis hospitalizations; and all with live discharge.
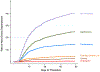
Measurements and main results: Among 18,210 pediatric severe sepsis hospitalizations, 1,024 (5.6%) underwent device placement. Specifically, 3.5% had new gastrostomy, 3.1% new tracheostomy, 0.6% new vascular access devices, 0.4% new ostomy procedures, and 0.1% amputations. One-hundred forty hospitalizations (0.8%) included two or more new devices. After applying the Nationwide Readmissions Database sampling weights, there were 55,624 pediatric severe sepsis hospitalizations and 1,585,194 all-cause nonsepsis hospitalizations with live discharge in 2016. Compared to all-cause pediatric hospitalizations, severe sepsis hospitalizations were eight-fold more likely to involve new device acquisition (6.4% vs 0.8%; p < 0.001). New device acquisition was also higher in severe sepsis hospitalizations compared with matched nonsepsis infection hospitalizations (5.1% vs 1.2%; p < 0.01) and matched all-cause hospitalizations with similar organ dysfunction (4.7% vs 2.8%; p < 0.001).
Conclusions: In this nationwide, all-payer cohort of U.S. pediatric severe sepsis hospitalizations, one in 20 children surviving severe sepsis experienced new device acquisition. The procedure rate was nearly eight-fold higher than all-cause, nonsepsis pediatric hospitalizations, and four-fold higher than matched nonsepsis infection hospitalizations.
Figures
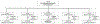
Similar articles
-
Readmission Diagnoses After Pediatric Severe Sepsis Hospitalization.Crit Care Med. 2019 Apr;47(4):583-590. doi: 10.1097/CCM.0000000000003646. Crit Care Med. 2019. PMID: 30676337 Free PMC article.
-
New and Progressive Medical Conditions After Pediatric Sepsis Hospitalization Requiring Critical Care.JAMA Pediatr. 2022 Nov 1;176(11):e223554. doi: 10.1001/jamapediatrics.2022.3554. Epub 2022 Nov 7. JAMA Pediatr. 2022. PMID: 36215045 Free PMC article.
-
Increased 1-year healthcare use in survivors of severe sepsis.Am J Respir Crit Care Med. 2014 Jul 1;190(1):62-9. doi: 10.1164/rccm.201403-0471OC. Am J Respir Crit Care Med. 2014. PMID: 24872085 Free PMC article.
-
Epidemiology of Readmissions After Sepsis Hospitalization in Children.Hosp Pediatr. 2019 Apr;9(4):249-255. doi: 10.1542/hpeds.2018-0175. Epub 2019 Mar 1. Hosp Pediatr. 2019. PMID: 30824488 Free PMC article.
-
Clinical Outcomes of Acute Myocardial Infarction Hospitalizations With Systemic Lupus Erythematosus: An Analysis of Nationwide Readmissions Database.Curr Probl Cardiol. 2022 Nov;47(11):101086. doi: 10.1016/j.cpcardiol.2021.101086. Epub 2021 Dec 20. Curr Probl Cardiol. 2022. PMID: 34936910 Review.
Cited by
-
Clinical study on the early systemic nursing care intervention in patients with severe pulmonary infection.Am J Transl Res. 2021 Apr 15;13(4):3745-3751. eCollection 2021. Am J Transl Res. 2021. PMID: 34017560 Free PMC article.
-
Association of an In-Hospital Desirability of Outcomes Ranking Scale With Postdischarge Health-Related Quality of Life: A Secondary Analysis of the Life After Pediatric Sepsis Evaluation.Pediatr Crit Care Med. 2024 Jun 1;25(6):528-537. doi: 10.1097/PCC.0000000000003470. Epub 2024 Feb 14. Pediatr Crit Care Med. 2024. PMID: 38353586 Free PMC article.
-
What's the Cost? Measuring the Economic Impact of Pediatric Sepsis.Front Pediatr. 2021 Nov 15;9:761994. doi: 10.3389/fped.2021.761994. eCollection 2021. Front Pediatr. 2021. PMID: 34869119 Free PMC article.
-
Biological basis of critical illness subclasses: from the bedside to the bench and back again.Crit Care. 2024 May 29;28(1):186. doi: 10.1186/s13054-024-04959-3. Crit Care. 2024. PMID: 38812006 Free PMC article. Review.
-
Pediatric Sepsis Diagnosis, Management, and Sub-phenotypes.Pediatrics. 2024 Jan 1;153(1):e2023062967. doi: 10.1542/peds.2023-062967. Pediatrics. 2024. PMID: 38084084 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

