Bile Biochemistry Following Liver Reperfusion in the Recipient and Its Association With Cholangiopathy
- PMID: 32108995
- PMCID: PMC7497270
- DOI: 10.1002/lt.25738
Bile Biochemistry Following Liver Reperfusion in the Recipient and Its Association With Cholangiopathy
Abstract
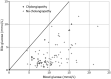
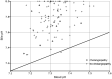
Cholangiocytes secrete bicarbonate and absorb glucose, producing bile with alkaline pH and low glucose content. These functions of cholangiocytes have been suggested as a marker of bile duct viability during normothermic ex situ liver perfusion, and they are now monitored routinely after reperfusion in our center. In this study, we reviewed the composition of bile immediately after reperfusion in liver transplant recipients to determine normal posttransplant parameters and the predictive value of bile biochemistry for the later development of cholangiopathy. After reperfusion of the liver graft, a cannula was placed in the bile duct to collect bile over a median 44-minute time period. The bile produced was analyzed using a point-of-care blood gas analyzer (Cobas b221, Roche Diagnostics, Indianapolis, IN). A total of 100 liver transplants (35 from donation after circulatory death and 65 from donation after brain death) were studied. Median bile pH was 7.82 (interquartile range [IQR], 7.67-7.98); median bile glucose was 2.1 (1.4-3.7) mmol/L; median blood-bile-blood pH difference was 0.50 (0.37-0.62); and median blood-bile glucose difference was 7.1 (5.6-9.1) mmol/L. There were 12 recipients who developed cholangiopathy over a median follow-up of 15 months (IQR, 11-20 months). Bile sodium (142 versus 147 mmol/L; P = 0.02) and blood-bile glucose concentration differences (5.2 versus 7.6 mmol/L; P = 0.001) were significantly lower and were associated with ischemic cholangiopathy. In conclusion, bile biochemistry may provide useful insights into cholangiocyte function and, hence, bile duct viability. Our results suggest bile glucose is the most sensitive predictor of cholangiopathy.
Copyright © 2020 The Authors. Liver Transplantation published by Wiley Periodicals, Inc., on behalf of American Association for the Study of Liver Diseases.
Figures

References
-
- Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, et al.; for Consortium for Organ Preservation in Europe . A randomized trial of normothermic preservation in liver transplantation. Nature 2018;557:50‐56. - PubMed
-
- Mergental H, Perera MT, Laing RW, Muiesan P, Isaac JR, Smith A, et al. Transplantation of declined liver allografts following normothermic ex‐situ evaluation. Am J Transplant 2016;16:3235‐3245. - PubMed
-
- Watson CJ, Kosmoliaptsis V, Randle LV, Russell NK, Griffiths WJ, Davies S, et al. Preimplant normothermic liver perfusion of a suboptimal liver donated after circulatory death. Am J Transplant 2016;16:353‐357. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

