Dual Plasmepsin-Targeting Antimalarial Agents Disrupt Multiple Stages of the Malaria Parasite Life Cycle
- PMID: 32109369
- PMCID: PMC7146544
- DOI: 10.1016/j.chom.2020.02.005
Dual Plasmepsin-Targeting Antimalarial Agents Disrupt Multiple Stages of the Malaria Parasite Life Cycle
Abstract
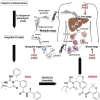
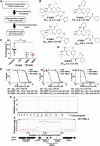
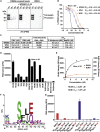
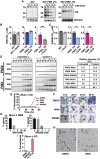
Artemisin combination therapy (ACT) is the main treatment option for malaria, which is caused by the intracellular parasite Plasmodium. However, increased resistance to ACT highlights the importance of finding new drugs. Recently, the aspartic proteases Plasmepsin IX and X (PMIX and PMX) were identified as promising drug targets. In this study, we describe dual inhibitors of PMIX and PMX, including WM382, that block multiple stages of the Plasmodium life cycle. We demonstrate that PMX is a master modulator of merozoite invasion and direct maturation of proteins required for invasion, parasite development, and egress. Oral administration of WM382 cured mice of P. berghei and prevented blood infection from the liver. In addition, WM382 was efficacious against P. falciparum asexual infection in humanized mice and prevented transmission to mosquitoes. Selection of resistant P. falciparum in vitro was not achievable. Together, these show that dual PMIX and PMX inhibitors are promising candidates for malaria treatment and prevention.
Keywords: Plasmodium; antimalarial; humanized mouse; malaria; merozoite; plasmepsin; plasmepsin IX; plasmepsin X.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests A.F.C., M.R., P.F., Z.G., Z.L., J.M., D.B.O., B.E.S., J.K.T, T.T., and L.Z. have a patent Antimalarial Agents PCT/CN2019/100781 based on the compounds in this manuscript.
Figures

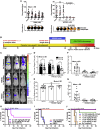
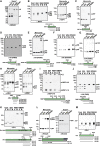
 . Molecular weight kDa.
. Molecular weight kDa.
 . C–F schematics show positions of F1 and F2 receptor binding domains. Molecular weight kDa.
. C–F schematics show positions of F1 and F2 receptor binding domains. Molecular weight kDa.Comment in
-
Targeting Plasmepsins-An Achilles' Heel of the Malaria Parasite.Cell Host Microbe. 2020 Apr 8;27(4):496-498. doi: 10.1016/j.chom.2020.03.015. Cell Host Microbe. 2020. PMID: 32272073
References
-
- Alaganan A., Singh P., Chitnis C.E. Molecular mechanisms that mediate invasion and egress of malaria parasites from red blood cells. Curr. Opin. Hematol. 2017;24:208–214. - PubMed
-
- Angulo-Barturen I., Jiménez-Díaz M.B., Mulet T., Rullas J., Herreros E., Ferrer S., Jiménez E., Mendoza A., Regadera J., Rosenthal P.J. A murine model of falciparum-malaria by in vivo selection of competent strains in non-myelodepleted mice engrafted with human erythrocytes. PLoS One. 2008;3:e2252. - PMC - PubMed
-
- Bacon D.J., Latour C., Lucas C., Colina O., Ringwald P., Picot S. Comparison of a SYBR green I-based assay with a histidine-rich protein II enzyme-linked immunosorbent assay for in vitro antimalarial drug efficacy testing and application to clinical isolates. Antimicrob. Agents Chemother. 2007;51:1172–1178. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

