Restraining Lysosomal Activity Preserves Hematopoietic Stem Cell Quiescence and Potency
- PMID: 32109377
- PMCID: PMC8075247
- DOI: 10.1016/j.stem.2020.01.013
Restraining Lysosomal Activity Preserves Hematopoietic Stem Cell Quiescence and Potency
Abstract
Quiescence is a fundamental property that maintains hematopoietic stem cell (HSC) potency throughout life. Quiescent HSCs are thought to rely on glycolysis for their energy, but the overall metabolic properties of HSCs remain elusive. Using combined approaches, including single-cell RNA sequencing (RNA-seq), we show that mitochondrial membrane potential (MMP) distinguishes quiescent from cycling-primed HSCs. We found that primed, but not quiescent, HSCs relied readily on glycolysis. Notably, in vivo inhibition of glycolysis enhanced the competitive repopulation ability of primed HSCs. We further show that HSC quiescence is maintained by an abundance of large lysosomes. Repression of lysosomal activation in HSCs led to further enlargement of lysosomes while suppressing glucose uptake. This also induced increased lysosomal sequestration of mitochondria and enhanced the competitive repopulation ability of primed HSCs by over 90-fold in vivo. These findings show that restraining lysosomal activity preserves HSC quiescence and potency and may be therapeutically relevant.
Keywords: HSC; dormancy; fission; hematopoietic stem cell; label retention; lysosomes; mTOR; mitochondria; mitophagy; quiescence.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests Some elements of this work have been filed in a patent application to the United States Patent and Trademark Office. R.L. is currently employed by HemoGenix.
Figures



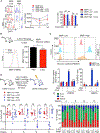
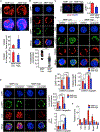


Comment in
-
Divide and Rule: Mitochondrial Fission Regulates Quiescence in Hematopoietic Stem Cells.Cell Stem Cell. 2020 Mar 5;26(3):299-301. doi: 10.1016/j.stem.2020.02.009. Cell Stem Cell. 2020. PMID: 32142657
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

