Modification of the host ubiquitome by bacterial enzymes
- PMID: 32109687
- PMCID: PMC7369425
- DOI: 10.1016/j.micres.2020.126429
Modification of the host ubiquitome by bacterial enzymes
Abstract
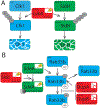
Attachment of ubiquitin molecules to protein substrates is a reversible post-translational modification (PTM), which occurs ubiquitously in eukaryotic cells and controls most cellular processes. As a consequence, ubiquitination is an attractive target of pathogen-encoded virulence factors. Pathogenic bacteria have evolved multiple mechanisms to hijack the host's ubiquitin system to their advantage. In this review, we discuss the bacteria-encoded E3 ligases and deubiquitinases translocated to the host for an addition or removal of eukaryotic ubiquitin modification, effectively hijacking the host's ubiquitination processes. We review bacterial enzymes homologous to host proteins in sequence and functions, as well as enzymes with novel mechanisms in ubiquitination, which have significant structural differences in comparison to the mammalian E3 ligases. Finally, we will also discuss examples of molecular "counter-weapons" - eukaryotic proteins, which counteract pathogen-encoded E3 ligases. The many examples of the pathogen effector molecules that catalyze eukaryotic ubiquitin modification bring to light the intricate pathways involved in the pathogenesis of some of the most virulent bacterial infections with human pathogens. The role of these effector molecules remains an essential determinant of bacterial virulence in terms of infection, invasion, and replication. A comprehensive understanding of the mechanisms dictating the mimicry employed by bacterial pathogens is of vital importance in developing new strategies for therapeutic approaches.
Keywords: Deubiquitinase; Host-Pathogen interactions; Ubiquitin; a Ubiquitin E3 ligase.
Copyright © 2020 Elsevier GmbH. All rights reserved.
Figures
References
-
- Arner ES, Holmgren A, 2000. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. biochemistry / FEBS 267 (20), 6102–6109. - PubMed
-
- Ashida H, Sasakawa C, 2017. Bacterial E3 ligase effectors exploit host ubiquitin systems. Curr. Opin. Microbiol 35, 16–22. - PubMed
-
- Ashida H, Toyotome T, Nagai T, Sasakawa C, 2007. Shigella chromosomal IpaH proteins are secreted via the type III secretion system and act as effectors. Mol. Microbiol 63 (3), 680–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

