PEGylated PLGA Nanoparticle Delivery of Eggmanone for T Cell Modulation: Applications in Rheumatic Autoimmunity
- PMID: 32110018
- PMCID: PMC7036983
- DOI: 10.2147/IJN.S234850
PEGylated PLGA Nanoparticle Delivery of Eggmanone for T Cell Modulation: Applications in Rheumatic Autoimmunity
Abstract
Background: Helper T cell activity is dysregulated in a number of diseases including those associated with rheumatic autoimmunity. Treatment options are limited and usually consist of systemic immune suppression, resulting in undesirable consequences from compromised immunity. Hedgehog (Hh) signaling has been implicated in the activation of T cells and the formation of the immune synapse, but remains understudied in the context of autoimmunity. Modulation of Hh signaling has the potential to enable controlled immunosuppression but a potential therapy has not yet been developed to leverage this opportunity.
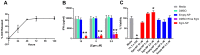
Methods: In this work, we developed biodegradable nanoparticles to enable targeted delivery of eggmanone (Egm), a specific Hh inhibitor, to CD4+ T cell subsets. We utilized two FDA-approved polymers, poly(lactic-co-glycolic acid) and polyethylene glycol, to generate hydrolytically degradable nanoparticles. Furthermore, we employed maleimide-thiol mediated conjugation chemistry to decorate nanoparticles with anti-CD4 F(ab') antibody fragments to enable targeted delivery of Egm.
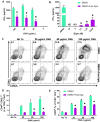
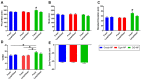
Results: Our novel delivery system achieved a highly specific association with the majority of CD4+ T cells present among a complex cell population. Additionally, we have demonstrated antigen-specific inhibition of CD4+ T cell responses mediated by nanoparticle-formulated Egm.
Conclusion: This work is the first characterization of Egm's immunomodulatory potential. Importantly, this study also suggests the potential benefit of a biodegradable delivery vehicle that is rationally designed for preferential interaction with a specific immune cell subtype for targeted modulation of Hh signaling.
Keywords: advanced delivery systems; autoimmunity; controlled release; eggmanone.
© 2020 Haycook et al.
Conflict of interest statement
Professor Charles C Hong and Dr Charles H Williams report a US Patent #: US 10,329,304 B2 issued. Professor Todd D Giorgio reports patent materials and methods for the treatment of immune cells pending. The authors report no other conflicts of interest in this work.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

