Alteration of Metal Elements in Radiation Injury: Radiation-Induced Copper Accumulation Aggravates Intestinal Damage
- PMID: 32110169
- PMCID: PMC7000859
- DOI: 10.1177/1559325820904547
Alteration of Metal Elements in Radiation Injury: Radiation-Induced Copper Accumulation Aggravates Intestinal Damage
Abstract
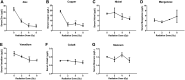
Ionizing radiation causes damage to a variety of tissues, especially radiation-sensitive tissues, such as the small intestine. Radiation-induced damage is caused primarily by increased oxidative stress in the body. Studies have shown that trace metal elements play an irreplaceable role in oxidative stress in humans, which may be associated with radiation-induced tissue damage. However, the alteration and functional significance of trace metal elements in radiation-induced injury is not clear. In this study, we explored the association between radiation-induced damage and 7 trace metal elements in mouse models. We found that the concentration of zinc and copper in mice serum was decreased significantly after irradiation, whereas that of nickel, manganese, vanadium, cobalt, and stannum was not changed by inductively coupled plasma mass spectrometry. The role of copper in radiation-induced intestines was characterized in detail. The concentration of copper was increased in irradiated intestine but reduced in irradiated heart. Immunohistochemistry staining showed that copper transporter protein copper transport 1 expression was upregulated in irradiated mouse intestine, suggesting its potential involvement in radiation-induced copper accumulation. At the cellular level, the addition of CuCl2 potentiated radiation-induced reactive oxygen species in intestine-derived human intestinal epithelial cell and IEC-6 cells. Moreover, the level of copper in damaged cells may be related to the severity of radiation-induced damage as evidenced by a cell viability assay. These results indicate that copper may be involved in the progression of radiation-induced tissue damage and may be a potential therapeutic target.
Keywords: copper; radiation; radiation-induced intestinal injury; trace metal element.
© The Author(s) 2020.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




References
-
- Burnet NG, Nyman J, Turesson I, Wurm R, Yarnold JR, Peacock JH. Prediction of normal-tissue tolerance to radiotherapy from in-vitro cellular radiation sensitivity. Lancet. 1992;339(8809):1570–1571. - PubMed
-
- Jomova K, Valko M, Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283(2-3):65–87. - PubMed
-
- Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev. 2015;95(3):749–784. - PubMed
LinkOut - more resources
Full Text Sources

