Sphere-forming corneal cells repopulate dystrophic keratoconic stroma: Implications for potential therapy
- PMID: 32110274
- PMCID: PMC7031758
- DOI: 10.4252/wjsc.v12.i1.35
Sphere-forming corneal cells repopulate dystrophic keratoconic stroma: Implications for potential therapy
Abstract
Background: Keratoconus is a degenerative corneal disease characterised by aberrant cell behaviour and loss of matrix that can result in vision loss. Cells extracted from peripheral corneas can form stem cell-enriched spheres, which have shown the potential to repopulate the normal peripheral corneal stroma in vitro upon sphere implantation but have not been previously studied in keratoconic tissue.
Aim: To investigate the therapeutic potential of stem cell-enriched spheres formed from extracted peripheral human corneal cells when introduced to keratoconic tissue.
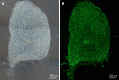
Methods: Stem cell-enriched spheres were formed from extracts of normal cadaveric human peripheral corneal cells. These spheres were implanted into incisions created in full thickness and onto the surface of 10 µm thin sections of keratoconic and normal stromal tissues in vitro. Tissue sections were used to maximise use of limited keratoconic tissue available for research. Living cells were stained with Calcein-AM and visualised with stereo and fluorescence microscopy to assess survival and behaviours between the time of implantation day 0 and 14 d (D14) from implantation. Sphere cells in implanted tissues were characterised for stem cell and differentiation markers using immunohistochemistry and droplet digital PCR to assess the potential implications of these characteristics in the use of spheres in keratoconus treatment.
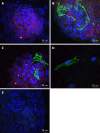
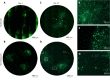
Results: Spheres were successfully implanted into full-thickness central corneal tissue and onto the surface of 10 µm thin en face tissue sections. No observable differences were seen in sphere migration, proliferation or differentiation in keratoconic tissue compared to normal between day 0 and D14. Spheres stained positively with Calcein-AM up to D14. Cell migration increased from day 0 to D14, occurring radially in three dimensions from the sphere and in alignment with tissue edges. Cell proliferation marker, EdU, was detected at day 10. Implanted spheres stained positively for putative stem cell markers ∆Np63α and ABCB5, while ABCG2, ABCB5, ∆Np63 and p63α were detectable by droplet digital PCR up to D14. Double immunolabelling revealed absence of ABCB5 staining in migrated cells but positive staining of alpha smooth muscle actin (myofibroblast marker) in some migrated cells. Droplet digital PCR showed similar expression patterns of differentiation markers but a reduction in stem cell markers between normal and keratoconic tissue with an increase in stromal cell markers and a reduction in epithelial cell markers, indicating an appropriate response to repopulating diseased tissue.
Conclusion: Cells from implanted stem cell-enriched spheres can repopulate a keratoconic corneal stromal surface in a directed manner and exhibit migratory stromal cell phenotypes.
Keywords: Cell culture; Digital PCR; Holoclone; Immunohistochemistry; Keratoconus; Neurosphere; Quantitative PCR; Regeneration; Spheroid.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Prof. Sherwin reports grants from Save Sight Society of New Zealand, grants from Auckland Medical Research Foundation, grants from New Zealand Wound Care Society, and grants from John Hamel MacGregor Trust during the conduct of the study.
Figures


References
-
- Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Cont Lens Anterior Eye. 2010;33:157–66; quiz 205. - PubMed
-
- Sherwin T, Brookes NH. Morphological changes in keratoconus: pathology or pathogenesis. Clin Exp Ophthalmol. 2004;32:211–217. - PubMed
-
- Somodi S, Hahnel C, Slowik C, Richter A, Weiss DG, Guthoff R. Confocal in vivo microscopy and confocal laser-scanning fluorescence microscopy in keratoconus. Ger J Ophthalmol. 1996;5:518–525. - PubMed
-
- Sherwin T, Brookes NH, Loh IP, Poole CA, Clover GM. Cellular incursion into Bowman's membrane in the peripheral cone of the keratoconic cornea. Exp Eye Res. 2002;74:473–482. - PubMed
-
- Kenney MC, Nesburn AB, Burgeson RE, Butkowski RJ, Ljubimov AV. Abnormalities of the extracellular matrix in keratoconus corneas. Cornea. 1997;16:345–351. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

