Two 3'- O-β-glucosylated nucleoside fluorometabolites related to nucleocidin in Streptomyces calvus
- PMID: 32110306
- PMCID: PMC7017864
- DOI: 10.1039/c9sc03374b
Two 3'- O-β-glucosylated nucleoside fluorometabolites related to nucleocidin in Streptomyces calvus
Abstract
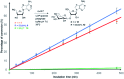
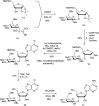
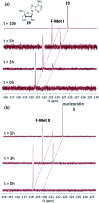
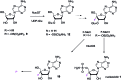
The antibiotic nucleocidin is a product of the soil bacterium Streptomyces calvus T-3018. It is among the very rare fluorine containing natural products but is distinct from the other fluorometabolites in that it is not biosynthesised from 5'-fluorodeoxyadenosine via the fluorinase. It seems to have a unique enzymatic fluorination process. We disclose here the structures of two 4'-fluoro-3'-O-β-glucosylated metabolites (F-Mets I and II) which appear and then disappear before nucleocidin production in batch cultures of S. calvus. Full genome sequencing of S. calvus T-3018 and an analysis of the putative biosynthetic gene cluster for nucleocidin identified UDP-glucose dependent glucosyl transferase (nucGT) and glucosidase (nucGS) genes within the cluster. We demonstrate that these genes express enzymes that have the capacity to attach and remove glucose from the 3'-O-position of adenosine analogues. In the case of F-Met II, deglucosylation with the NucGS glucosidase generates nucleocidin suggesting a role in its biosynthesis. Gene knockouts of nucGT abolished nucelocidin production.
This journal is © The Royal Society of Chemistry 2019.
Figures



References
-
- O'Hagan D., Deng H. Chem. Rev. 2015;115:634–649. - PubMed
-
- Thomas S. O., Singleton V. L., Lowery J. A., Sharpe R. W., Pruess L. M., Porter J. N., Mowat J. H., Bohonos N. Antibiot. Annu. 1956:1956–1957. - PubMed
-
- Isono K., Uramoto M., Kusakabe H., Miyata N., Koyama T. J. Antibiot. 1984;37:670–672. - PubMed
- Takahashi E., Beppu T. Biochem. J. 1982;233:459–463.
-
- Sanada M., Miyano T., Iwadare S., Williamson J. M., Arison B. H., Smith J. L., Douglas A. W., Leich J. M., Inamine E. J. Antibiot. 1986;39:259–265. - PubMed
-
- Deng H., Ma L., Bandaranayaka N., Qin Z., Mann G., Kyeremeh K., Yu Y., Shephers T., Naismith J. H., O'Hagan D. ChemBioChem. 2014;15:364–368. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

