Cu-catalyzed C-C bond formation of vinylidene cyclopropanes with carbon nucleophiles
- PMID: 32110346
- PMCID: PMC7020789
- DOI: 10.1039/c9sc04122b
Cu-catalyzed C-C bond formation of vinylidene cyclopropanes with carbon nucleophiles
Erratum in
-
Correction: Cu-catalyzed C-C bond formation of vinylidene cyclopropanes with carbon nucleophiles.Chem Sci. 2020 Jan 10;11(4):1177. doi: 10.1039/c9sc90262g. eCollection 2020 Jan 28. Chem Sci. 2020. PMID: 35414862 Free PMC article.
Abstract
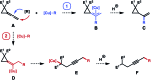
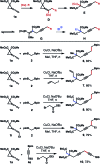
The development of Cu-catalyzed addition of carbon nucleophiles to vinylidene cyclopropanes was reported. The reactions with 1,1-bisborylmethane provided homopropargylic boronate products by forming a C-C bond at the terminal carbon atom of the allene moiety of vinylidene cyclopropanes. Alkynyl boronates are also suitable nucleophile precursors in reactions with vinylidene cyclopropanes, and skipped diynes were obtained in high yields. In addition, the Cu-enolate generated from the initial addition of nucleophilic copper species to vinylidene cyclopropanes can be intercepted by an external electrophile. As such, vinylidene cyclopropane serves as a linchpin to connect a nucleophile and an electrophile by forming two carbon-carbon bonds sequentially.
This journal is © The Royal Society of Chemistry 2019.
Figures
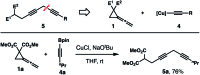

References
-
- Campbell M. J., Pohlhaus P. D., Min G., Ohmatsu K., Johnson J. S. J. Am. Chem. Soc. 2008;130:9180. - PubMed
-
-
For selected recent references with vinylidene cyclopropanes, see:
- Yang S., Rui K.-H., Tang X.-Y., Xu Q., Shi M. J. Am. Chem. Soc. 2017;139:5957. - PubMed
- Ji C., Xu Q., Shi M. Adv. Synth. Catal. 2017;359:974.
- Yuan W., Dong X., Shi M., McDowell P., Li G. Org. Lett. 2012;14:5582. - PMC - PubMed
- Wu L., Shi M. Chem.–Eur. J. 2011;17:13160. - PubMed
- Wu L., Shi M. Eur. J. Org. Chem. 2011;2011:1099.
- Wu L., Shi M. Chem.–Eur. J. 2010;16:1149. - PubMed
- Wu L., Shi M. J. Org. Chem. 2010;75:2296. - PubMed
-
-
-
For selected recent references on Cu-catalyzed reactions of allene with boron nucleophiles, see:
- Semba K., Fujihara T., Terao J., Tsuji Y. Angew. Chem., Int. Ed. 2013;52:12400. - PubMed
- Semba K., Shinomiya M., Fujihara T., Terao J., Tsuji Y. Chem.–Eur. J. 2013;19:7125. - PubMed
- Yuan W., Zhang X., Yu Y., Ma S. Chem.–Eur. J. 2013;19:7193. - PubMed
- Meng F., McGrath K. P., Hoveyda A. H. Nature. 2014;513:367. - PMC - PubMed
- Semba K., Bessho N., Fujihara T., Terao J., Tsuji Y. Angew. Chem., Int. Ed. 2014;53:9007. - PubMed
- Meng F., Li X., Torker S., Shi Y., Shen X., Hoveyda A. H. Nature. 2016;537:387. - PMC - PubMed
- Zhao W., Montgomery J. J. Am. Chem. Soc. 2016;138:9763. - PubMed
- Zhao J., Szabó K. J. Angew. Chem., Int. Ed. 2016;55:1502. - PMC - PubMed
- Yeung K., Ruscoe R. E., Rae J., Pulis A. P., Procter D. J. Angew. Chem., Int. Ed. 2016;55:11912. - PMC - PubMed
- Huang Y., Torker S., Li X., del Pozo J., Hoveyda A. H. Angew. Chem., Int. Ed. 2019;58:2685. - PMC - PubMed
-
LinkOut - more resources
Full Text Sources
Research Materials

