Synthesis of bicyclo[3.1.0]hexanes by (3 + 2) annulation of cyclopropenes with aminocyclopropanes
- PMID: 32110351
- PMCID: PMC7006509
- DOI: 10.1039/c9sc03790j
Synthesis of bicyclo[3.1.0]hexanes by (3 + 2) annulation of cyclopropenes with aminocyclopropanes
Abstract
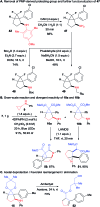
We report the convergent synthesis of bicyclo[3.1.0]hexanes possessing an all-carbon quaternary center via a (3 + 2) annulation of cyclopropenes with cyclopropylanilines. Using an organic or an iridium photoredox catalyst and blue LED irradiation, good yields were obtained for a broad range of cyclopropene and cyclopropylaniline derivatives. The reaction was highly diastereoselective when using difluorocyclopropenes together with a removable substituent on the cyclopropylaniline, giving access to important building blocks for medicinal chemistry. With efficient methods existing for the synthesis of both reaction partners, our method grants a fast access to highly valuable bicyclic scaffolds with three contiguous stereocenters.
This journal is © The Royal Society of Chemistry 2019.
Figures





References
-
- Kim H. S., Ohno M., Xu B., Kim H. O., Choi Y. S., Ji X. D., Maddileti S., Marquez V. E., Harden T. K., Jacobson K. A. J. Med. Chem. 2003;46:4974. - PMC - PubMed
- Parks J., Gyeltshen T., Prachyawarakorn V., Mahidol C., Ruchirawat S., Kittakoop P. J. Nat. Prod. 2010;73:992. - PubMed
- Boatman P. D., Lauring B., Schrader T. O., Kasem M., Johnson B. R., Skinner P., Jung J. K., Xu J., Cherrier M. C., Webb P. J., Semple G., Sage C. R., Knudsen J., Chen R., Luo W. L., Caro L., Cote J., Lai E., Wagner J., Taggart A. K., Carballo-Jane E., Hammond M., Colletti S. L., Tata J. R., Connolly D. T., Waters M. G., Richman J. G. J. Med. Chem. 2012;55:3644. - PubMed
- Liu M. L., Duan Y. H., Hou Y. L., Li C., Gao H., Dai Y., Yao X. S. Org. Lett. 2013;15:1000. - PubMed
-
- Fenical W., Sims J. J. Tetrahedron Lett. 1974;15:1137.
- Ireland C., Faulkner J. Tetrahedron. 1981;37:233.
- Garcia-Davis S., Viveros-Valdez E., Diaz-Marrero A. R., Fernández J. J., Valencia-Mercado D., Esquivel-Hernández O., Carranza-Rosales P., Carranza-Torres I. E., Guzman-Delgado N. E. Mar. Drugs. 2019;17:201. - PMC - PubMed
-
- Monn J. A., Valli M. J., Massey S. M., Wright R. A., Salhoff C. R., Johnson B. G., Howe T., Alt C. A., Rhodes G. A., Robey R. L., Griffey K. R., Tizzano J. P., Kallman M. J., Helton D. R., Schoepp D. D. J. Med. Chem. 1997;40:528. - PubMed
-
- Csuk R., Heinold A., Siewert B., Schwarz S., Barthel A., Kluge R., Ströhl D. Arch. Pharm. 2012;345:215. - PubMed
-
- Wang Z., Silverman R. B. Bioorg. Med. Chem. 2006;14:2242. - PubMed
LinkOut - more resources
Full Text Sources

