Severe cutaneous adverse drug reactions manifesting as Stevens-Johnson syndrome and toxic epidermal necrolysis reported to the national pharmacovigilance center in Nigeria: a database review from 2004 to 2017
- PMID: 32110375
- PMCID: PMC7016315
- DOI: 10.1177/2042098620905998
Severe cutaneous adverse drug reactions manifesting as Stevens-Johnson syndrome and toxic epidermal necrolysis reported to the national pharmacovigilance center in Nigeria: a database review from 2004 to 2017
Abstract
Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe cutaneous adverse reactions (SCARs). There is scant literature on the characteristics and causes of these conditions among the Nigerian population. Here, we describe the epidemiology, associated morbidity and mortality, and culpable drugs in SJS and TEN cases using the National Pharmacovigilance (NPC) database in Nigeria.
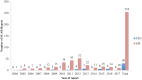
Methods: A retrospective review of the NPC database was done to analyze SJS and TEN cases reported over a period of 14 years. Annual reports, age and sex of patients, type of reporter, suspects and concomitant drugs, time to onset (TTO) of the reactions, and outcome of SJS and TEN were evaluated.
Results: The NPC received a total of 24,015 adverse drug reaction (ADR) reports. SJS and TEN accounted for 284 (0.1%) of the total reports, of which 254 (89.4%) were SJS and the remainder were TEN. Females (n = 184, 64.8%) and individuals aged 19-40 years (n = 181, 63.7%) were the most affected by SJS and TEN. Antiretrovirals, followed by antibiotics, were the most common drug classes reported to cause SJS and TEN, with nevirapine (n = 174, 40.7%) and co-trimoxazole (n = 143, 33.5%) being the most widely implicated drugs. Among patients with reported outcomes, 73 (28.7%) SJS and 3 (10.0%) TEN cases recovered without sequelae, at the time of reporting. Severity of the SCAR was reported for only 171 (69.0%) cases, of which 12 (4.7%) and 8 (26.7%) resulted in death (Grade 5) among SJS and TEN cases, respectively.
Conclusions: Antiretroviral and antibiotics were the commonly reported offending group of drugs for SJS and TEN cases. Nevirapine and co-trimoxazole were the commonly reported suspect drugs. SJS and TEN were reported most frequently in females and in patients aged 19-40 years, indicating that drug surveillance and counseling in these groups of patients may be beneficial.
Keywords: Nigeria; Severe; Stevens- Johnson syndrome; adverse drug reactions; database; pharmacovigilance; toxic epidermal necrolysis.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures

References
-
- Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother 2008; 42: 1017–1025. - PubMed
-
- Marzano AV, Borghi A, Cugno M. Adverse drug reactions and organ damage: the skin. Eur J Intern Med 2016; 28: 17–24. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

