Trends in FDA drug approvals over last 2 decades: An observational study
- PMID: 32110574
- PMCID: PMC7014862
- DOI: 10.4103/jfmpc.jfmpc_578_19
Trends in FDA drug approvals over last 2 decades: An observational study
Abstract
Introduction: The discovery of novel drugs is critical for pharmaceutical research and development as well as for patient treatment. Repurposing existing drugs that may have anticipated effects as potential candidate is one way to meet this important goal. Systematic investigation and comprehensive analysis of approved drugs could provide valuable insights into trends in the discovery and may contribute to further discovery of newer drugs systematically. Food and drug administration (FDA's) Center for Drug Evaluation and Research (CDER) every year summarizes novel drugs, some of which are truly innovative and help in advancing clinical care. This study was conducted to find a trend in drug approvals by FDA in the last 2 decades. Awareness of these new drugs amongst the primary care physicians is also crucial as they have been prescribing these agents in the past.
Methodology: In this cross-sectional study, we collected, surveyed, and analyzed drugs approved by U.S. Food and Drug Administration (USFDA) from the year 2000 till 2017 identified from ClinicalTrials.gov and online database of FDA. Drugs approved every year were assessed for total number, class of drug, indication, and category of approval. Type of accelerated regulatory pathways and reasons for speedy approvals every year were also studied. Microsoft Office Excel 2007 was used for tabulation and analysis.
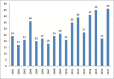
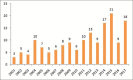
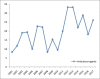
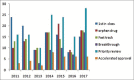
Results: Total 209 were approved from 2000 to 2008. Out of these 9.09% were indicated for cardiovascular disorders and 12.91% for neurological disorders. Antibiotics (5.26%) and antivirals (5.74%) were least contributed, whereas anticancer drugs (11.96%) and biologics (7.17%) approval remained constant. Whereas, out of three hundred and two drugs approved during 2009--2017, 5.29% were for cardiovascular disorders, 9.93% for neurological disorders. Antibiotics (5.29%) and antivirals (5.96%) were least in number, whereas anticancer drugs (17.54%) and biologics (15.56%) approval took a steep rise in these years. Also, a wide variation in the number and category of approval was observed over a period of years. The use of fast track, accelerated approval, and priority review programs have also been steadily increasing since 2000.
Conclusion: There has been a steady rate of introduction of new drugs by CDER over the last two decades. Expedited approval of anticancer and biologics is seen as recent trend in drug development. Relatively, slow progress in approval of drugs for neurological disorders (depression, psychosis, multiple sclerosis, etc.) and lifestyle diseases like obesity, atherosclerosis, diabetes, etc., were seen. These findings reflect more emphasis being laid down in research for anticancer drugs and biologics.
Keywords: Drug approval; USFDA; drug discovery and development.
Copyright: © 2020 Journal of Family Medicine and Primary Care.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Meadows M. Promoting safe and effective drugs for 100 years. FDA Consumer Magazine. FDA Consum. 2006;40:14–20. - PubMed
-
- US Food and Drug Administration. Milestones of drug regulation in the United States. 2012 [Online] 2018. Jan 02, cited 2018 Dec 11. Available from: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm2007256.htm .
-
- Williams CT. Food and drug administration drug approval process: A history and overview. Nurs Clin. 2016;51:1–11. - PubMed
-
- Schwieterman WD. Regulating biopharmaceuticals under CDER versus CBER: An insider's perspective. Drug Discov Today. 2006;11:945–51. - PubMed
-
- Kaitin KI, DiMasi JA. Pharmaceutical innovation in the 21st century: New drug approvals in the first decade, 2000–2009. Clin Pharm Ther. 2011;89:183–8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous