A helical inner scaffold provides a structural basis for centriole cohesion
- PMID: 32110738
- PMCID: PMC7021493
- DOI: 10.1126/sciadv.aaz4137
A helical inner scaffold provides a structural basis for centriole cohesion
Abstract
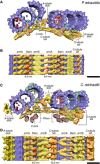
The ninefold radial arrangement of microtubule triplets (MTTs) is the hallmark of the centriole, a conserved organelle crucial for the formation of centrosomes and cilia. Although strong cohesion between MTTs is critical to resist forces applied by ciliary beating and the mitotic spindle, how the centriole maintains its structural integrity is not known. Using cryo-electron tomography and subtomogram averaging of centrioles from four evolutionarily distant species, we found that MTTs are bound together by a helical inner scaffold covering ~70% of the centriole length that maintains MTTs cohesion under compressive forces. Ultrastructure Expansion Microscopy (U-ExM) indicated that POC5, POC1B, FAM161A, and Centrin-2 localize to the scaffold structure along the inner wall of the centriole MTTs. Moreover, we established that these four proteins interact with each other to form a complex that binds microtubules. Together, our results provide a structural and molecular basis for centriole cohesion and geometry.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- Abal M., Keryer G., Bornens M., Centrioles resist forces applied on centrosomes during G2/M transition. Biol. Cell 97, 425–434 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

