Solvent-Free Iron(III) Chloride-Catalyzed Direct Amidation of Esters
- PMID: 32110915
- PMCID: PMC7179140
- DOI: 10.3390/molecules25051040
Solvent-Free Iron(III) Chloride-Catalyzed Direct Amidation of Esters
Abstract
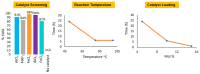
Amide functional groups are prominent in a broad range of organic compounds with diverse beneficial applications. In this work, we report the synthesis of these functional groups via an iron(iii) chloride-catalyzed direct amidation of esters. The reactions are conducted under solvent-free conditions and found to be compatible with a range of amine and ester substrates generating the desired amides in short reaction times and good to excellent yields at a catalyst loading of 15 mol%.
Keywords: amidation; amides; esters; iron(III) chloride; solvent-free.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

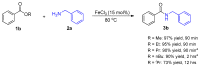

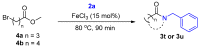

Similar articles
-
Amide synthesis from esters with nitriles under solvent-free conditions using molecular iodine as a catalyst.J Oleo Sci. 2012;61(7):393-9. doi: 10.5650/jos.61.393. J Oleo Sci. 2012. PMID: 22790170
-
Computational Study of Mechanism and Thermodynamics of Ni/IPr-Catalyzed Amidation of Esters.Molecules. 2018 Oct 18;23(10):2681. doi: 10.3390/molecules23102681. Molecules. 2018. PMID: 30340335 Free PMC article.
-
Manganese Catalyzed Direct Amidation of Esters with Amines.J Org Chem. 2021 Feb 5;86(3):2339-2358. doi: 10.1021/acs.joc.0c02478. Epub 2021 Jan 7. J Org Chem. 2021. PMID: 33411529
-
Non-conventional hydrolase chemistry: amide and carbamate bond formation catalyzed by lipases.Bioorg Med Chem. 1999 Oct;7(10):2189-97. doi: 10.1016/s0968-0896(99)00150-9. Bioorg Med Chem. 1999. PMID: 10579525 Review.
-
Well-Defined Pre-Catalysts in Amide and Ester Bond Activation.Molecules. 2019 Jan 9;24(2):215. doi: 10.3390/molecules24020215. Molecules. 2019. PMID: 30634382 Free PMC article. Review.
Cited by
-
Stereocontrolled synthesis of 3-hydroxy-2-piperidinone carboxamides by catalytic ring-opening aminolysis of bridged δ-lactam-γ-lactones.RSC Adv. 2025 May 14;15(20):16028-16034. doi: 10.1039/d5ra02161h. eCollection 2025 May 12. RSC Adv. 2025. PMID: 40370848 Free PMC article.
-
6-Halo-2-pyridone as an efficient organocatalyst for ester aminolysis.RSC Adv. 2021 Jul 14;11(40):24588-24593. doi: 10.1039/d1ra04651a. eCollection 2021 Jul 13. RSC Adv. 2021. PMID: 35481026 Free PMC article.
References
-
- Ponomaryov Y., Krasikova V., Lebedev A., Chernyak D., Varacheva L., Chernobroviy A. Scalable and green process for the synthesis of anticancer drug lenalidomide. Chem. Heterocycl. Comp. 2015;51:133–138. doi: 10.1007/s10593-015-1670-0. - DOI
-
- Zhou A.-N., Li B., Ruan L., Wang Y., Duan G., Li J. An improved and practical route for the synthesis of enzalutamide and potential impurities study. Chin. Chem. Lett. 2017;28:426–430. doi: 10.1016/j.cclet.2016.09.007. - DOI
-
- Volovych I., Neumann M., Schmidt M., Buchner G., Yang J.-Y., Wölk J., Sottmann T., Strey R., Schomäcker R., Schwarze M. A novel process concept for the three step Boscalid® synthesis. RSC Adv. 2016;6:58279–58287. doi: 10.1039/C6RA10484C. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

