Application of Halogen Bonding to Organocatalysis: A Theoretical Perspective
- PMID: 32110944
- PMCID: PMC7179134
- DOI: 10.3390/molecules25051045
Application of Halogen Bonding to Organocatalysis: A Theoretical Perspective
Abstract
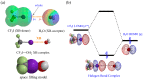
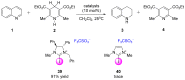
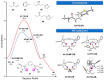
The strong, specific, and directional halogen bond (XB) is an ideal supramolecular synthon in crystal engineering, as well as rational catalyst and drug design. These attributes attracted strong growing interest in halogen bonding in the past decade and led to a wide range of applications in materials, biological, and catalysis applications. Recently, various research groups exploited the XB mode of activation in designing halogen-based Lewis acids in effecting organic transformation, and there is continual growth in this promising area. In addition to the rapid advancements in methodology development, computational investigations are well suited for mechanistic understanding, rational XB catalyst design, and the study of intermediates that are unstable when observed experimentally. In this review, we highlight recent computational studies of XB organocatalytic reactions, which provide valuable insights into the XB mode of activation, competing reaction pathways, effects of solvent and counterions, and design of novel XB catalysts.
Keywords: density functional theory (DFT); halogen bond; mechanism; noncovalent interaction; organocatalysis; supramolecular chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
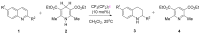
















References
-
- Desiraju G.R., Ho P.S., Kloo L., Legon A.C., Marquardt R., Metrangolo P., Politzer P., Resnati G., Rissanen K. Definition of the halogen bond (IUPAC Recommendations 2013) Pure Appl. Chem. 2013;85:1711–1713. doi: 10.1351/PAC-REC-12-05-10. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

