Antimicrobial, Antioxidant, and Anticancer Activities of Some Novel Isoxazole Ring Containing Chalcone and Dihydropyrazole Derivatives
- PMID: 32110945
- PMCID: PMC7179112
- DOI: 10.3390/molecules25051047
Antimicrobial, Antioxidant, and Anticancer Activities of Some Novel Isoxazole Ring Containing Chalcone and Dihydropyrazole Derivatives
Abstract
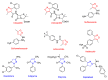
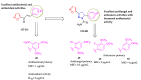
Our previous work identified isoxazole-based chalcones and their dihydropyrazole derivatives as two important five-membered heterocycles having antitubercular activity. Hence, in the present study, we biologically evaluated 30 compounds, including 15 isoxazole ring-containing chalcones (17-31) and 15 dihydropyrazoles (32-46) derived from these chalcones for their antimicrobial, antioxidant, and anticancer activities. Chalcones exhibited superior antibacterial and antioxidant activities compared to dihydropyrazoles. Among the chalcones, compound 28 showed potent antibacterial (MIC = 1 µg/mL) and antioxidant activities (IC50 = 5 ± 1 µg/mL). Dihydropyrazoles, on the contrary, demonstrated remarkable antifungal and anticancer activities. Compound 46 (IC50 = 2 ± 1 µg/mL) showed excellent antifungal activity whereas two other dihydropyrazoles 45 (IC50 = 2 ± 1 µg/mL) and 39 (IC50 = 4 ± 1 µg/mL) exhibited potential anticancer activity. The compounds were also tested for their toxicity on normal human cell lines (LO2) and were found to be nontoxic. The active compounds that have emerged out of this study are potential lead molecules for the development of novel drugs against infectious diseases, oxidative stress, and cancer.
Keywords: antibacterial activity; anticancer activity; antifungal activity; chalcones; dihydropyrazole; isoxazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gao Z., Hurst W.J., Czechtizky W., Hall D., Moindrot N., Nagorny R., Pichat P., Stefany D., Hendrix J.A., George P.G. Identification and profiling of 3,5-dimethyl-isoxazole-4-carboxylic acid [2-methyl-4-((2S,3′S)-2-methyl-[1,3′]bipyrrolidinyl-1′-yl)phenyl] amide as histamine H(3) receptor antagonist for the treatment of depression. Bioorg. Med. Chem. Lett. 2013;23:6269–6273. doi: 10.1016/j.bmcl.2013.09.081. - DOI - PubMed
-
- Afzal B.S., Lohitha S.V.K., Puttagunta S.B., Shaik A., Supraja K., Sai H.K. Synthesis and screening of novel lipophilic diarylpropeones as prospective antitubercular, antibacterial and antifungal agents. Biointerface Res. Appl. Chem. 2019;9:3912–3918.
-
- RamirezPrada J., Robledo S.M., Velez I.D., Crespo M.D.P., Quiroga J., Abonia R., Montoya A., Svetaz L., Zacchino S., Insuasty B. Synthesis of novel quinoline-based 4,5-dihydro-1-H-pyrazoles as potential anticancer, antifungal, antibacterial, antiprotozoal agents. Med. Chem. 2017;131:237–254. - PubMed
-
- Sowmya D.V., Lakshmi Teja A., Padmaja A., Kamala Prasad V., Padmavathi V. Green approach for the synthesis of thiophenyl pyrazoles and isoxazoles by adopting 1,3-dipolar cycloaddition methodology and their antimicrobial activity. Eur. J. Med. Chem. 2018;143:891–898. doi: 10.1016/j.ejmech.2017.11.093. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

