Initiation of Conceptus Elongation Coincides with an Endometrium Basic Fibroblast Growth Factor (FGF2) Protein Increase in Heifers
- PMID: 32111034
- PMCID: PMC7084457
- DOI: 10.3390/ijms21051584
Initiation of Conceptus Elongation Coincides with an Endometrium Basic Fibroblast Growth Factor (FGF2) Protein Increase in Heifers
Abstract
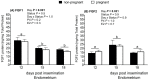
Fibroblast growth factors (FGF) play an important role during embryo development. To date, the role of FGF and the respective receptors (FGFR) during the preimplantation phase in cattle are not fully characterized. We examined FGF1, FGF2, FGFR1, FGFR2, and FGFR3 in cyclic and early pregnant heifers at Days 12, 15, and 18 after insemination (Day 0). Endometrial FGF1 mRNA transcript abundance in heifers varied significantly with respect to the day after insemination, the pregnancy status, and their interaction. The expression was higher in nonpregnant than in pregnant heifers at Day 18. The conceptus transcripts abundance of FGFR2 and FGFR3 were significantly lower at Day 15 than 18. In the endometrium, FGF1 protein abundance significantly decreased from Day 12 onwards and FGF2 protein abundance showed a minor, but a significant increase at Day 15 in comparison to Days 12 and 18. We concluded that the decrease in FGF1 mRNA expression in pregnant heifers at Day 18 points towards a potential contribution of FGF1 in the preimplantation process. Additionally, successful embryo elongation might require a spatiotemporal FGF2 protein increase in the endometrium.
Keywords: FGF2; angiogenesis; bovine; embryo; interferon-tau.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Fibroblast growth factor-2 expression in the preimplantation equine conceptus and endometrium of pregnant and cyclic mares.Theriogenology. 2013 Dec;80(9):979-89. doi: 10.1016/j.theriogenology.2013.07.024. Epub 2013 Sep 12. Theriogenology. 2013. PMID: 24035195
-
Ovine endometrial expression of fibroblast growth factor (FGF) 2 and conceptus expression of FGF receptors during early pregnancy.Domest Anim Endocrinol. 2008 Feb;34(2):135-45. doi: 10.1016/j.domaniend.2006.12.002. Epub 2007 Jan 3. Domest Anim Endocrinol. 2008. PMID: 17223006
-
Temporal regulation of fibroblast growth factors and their receptors in the endometrium and conceptus during the pre-implantation period of pregnancy in cattle.Reproduction. 2014 Jun;147(6):825-34. doi: 10.1530/REP-13-0373. Epub 2014 Feb 19. Reproduction. 2014. PMID: 24554351
-
Symposium review: Immunological detection of the bovine conceptus during early pregnancy.J Dairy Sci. 2019 Apr;102(4):3766-3777. doi: 10.3168/jds.2018-15668. Epub 2019 Feb 1. J Dairy Sci. 2019. PMID: 30712941 Review.
-
[Control of the intracellular signaling induced by fibroblast growth factors (FGF) over the proliferation and survival of retinal pigment epithelium cells: example of the signaling regulation of growth factors endogenous to the retina ].J Soc Biol. 2001;195(2):101-6. J Soc Biol. 2001. PMID: 11723820 Review. French.
Cited by
-
Predicting of molecules mediating an interaction between bovine embryos and uterine epithelial cells.J Reprod Dev. 2022 Oct 6;68(5):318-323. doi: 10.1262/jrd.2022-046. Epub 2022 Jul 29. J Reprod Dev. 2022. PMID: 35908976 Free PMC article.
-
Single-Cell Transcriptomics of Proliferative Phase Endometrium: Systems Analysis of Cell-Cell Communication Network Using CellChat.Front Cell Dev Biol. 2022 Jul 22;10:919731. doi: 10.3389/fcell.2022.919731. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35938159 Free PMC article.
-
Embryo-Maternal Interactions Underlying Reproduction in Mammals.Int J Mol Sci. 2020 Jul 10;21(14):4872. doi: 10.3390/ijms21144872. Int J Mol Sci. 2020. PMID: 32664189 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

