Anti-Pituitary and Anti-Hypothalamus Autoantibody Associations with Inflammation and Persistent Hypogonadotropic Hypogonadism in Men with Traumatic Brain Injury
- PMID: 32111134
- PMCID: PMC7336882
- DOI: 10.1089/neu.2019.6780
Anti-Pituitary and Anti-Hypothalamus Autoantibody Associations with Inflammation and Persistent Hypogonadotropic Hypogonadism in Men with Traumatic Brain Injury
Abstract
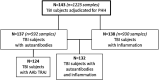
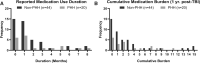
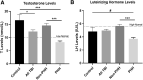
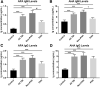
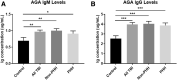
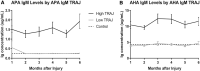
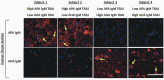
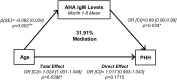
Traumatic brain injury (TBI) and can lead to persistent hypogonadotropic hypogonadism (PHH) and poor outcomes. We hypothesized that autoimmune and inflammatory mechanisms contribute to PHH pathogenesis. Men with moderate-to-severe TBI (n = 143) were compared with healthy men (n = 39). The TBI group provided blood samples 1-12 months post-injury (n = 1225). TBI and healthy control (n = 39) samples were assayed for testosterone (T) and luteinizing hormone (LH) to adjudicate PHH status. TBI samples 1-6 months post-injury and control samples were assayed for immunoglobulin M (IgM)/immunoglobulin G (IgG) anti-pituitary autoantibodies (APA) and anti-hypothalamus autoantibodies (AHA). Tissue antigen specificity for APA and AHA was confirmed via immunohistochemistry (IHC). IgM and IgG autoantibodies for glial fibrillary acid protein (GFAP) (AGA) were evaluated to gauge APA and AHA production as a generalized autoimmune response to TBI and to evaluate the specificity of APA and AHA to PHH status. An inflammatory marker panel was used to assess relationships to autoantibody profiles and PHH status. Fifty-one men with TBI (36%) had PHH. An age-related decline in T levels by both TBI and PHH status were observed. Injured men had higher APA IgM, APA IgG, AHA IgM, AHA IgG, AGA IgM, and AGA IgG than controls (p < 0.0001 all comparisons). However, only APA IgM (p = 0.03) and AHA IgM (p = 0.03) levels were lower in the PHH than in the non-PHH group in multivariate analysis. There were no differences in IgG levels by PHH status. Multiple inflammatory markers were positively correlated with IgM autoantibody production. PHH was associated with higher soluble tumor-necrosis-factor receptors I/II, (sTNFRI, sTNFRII), regulated on activation, normal T-cell expressed and secreted (RANTES) and soluble interleukin-2-receptor-alpha (sIL-2Rα) levels. Higher IgM APA, and AHA, but not AGA, in the absence of PHH may suggest a beneficial or reparative role for neuroendocrine tissue-specific IgM autoantibody production against PHH development post-TBI.
Keywords: IgG autoantibody; IgM autoantibody; TBI; autoantibodies; autoimmunity; hypogonadism; hypopituitarism; inflammation.
Conflict of interest statement
No competing financial interests exist.
Figures



Similar articles
-
Persistent Hypogonadotropic Hypogonadism in Men After Severe Traumatic Brain Injury: Temporal Hormone Profiles and Outcome Prediction.J Head Trauma Rehabil. 2016 Jul-Aug;31(4):277-87. doi: 10.1097/HTR.0000000000000188. J Head Trauma Rehabil. 2016. PMID: 26360007 Free PMC article.
-
Persistent hypogonadism influences estradiol synthesis, cognition and outcome in males after severe TBI.Brain Inj. 2012;26(10):1226-42. doi: 10.3109/02699052.2012.667594. Epub 2012 May 9. Brain Inj. 2012. PMID: 22571223
-
Plasma Anti-Glial Fibrillary Acidic Protein Autoantibody Levels during the Acute and Chronic Phases of Traumatic Brain Injury: A Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot Study.J Neurotrauma. 2016 Jul 1;33(13):1270-7. doi: 10.1089/neu.2015.3881. Epub 2016 Feb 1. J Neurotrauma. 2016. PMID: 26560343 Free PMC article.
-
The role of autoimmunity in pituitary dysfunction due to traumatic brain injury.Pituitary. 2019 Jun;22(3):236-248. doi: 10.1007/s11102-019-00953-z. Pituitary. 2019. PMID: 30847776 Review.
-
Traumatic Brain Injury as Frequent Cause of Hypopituitarism and Growth Hormone Deficiency: Epidemiology, Diagnosis, and Treatment.Front Endocrinol (Lausanne). 2021 Mar 15;12:634415. doi: 10.3389/fendo.2021.634415. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33790864 Free PMC article. Review.
Cited by
-
Acute neuroendocrine changes after traumatic brain injury.Brain Spine. 2024 May 7;4:102830. doi: 10.1016/j.bas.2024.102830. eCollection 2024. Brain Spine. 2024. PMID: 38764890 Free PMC article. Review.
-
Beyond Binary: Influence of Sex and Gender on Outcome after Traumatic Brain Injury.J Neurotrauma. 2020 Dec 1;37(23):2454-2459. doi: 10.1089/neu.2020.7230. Epub 2020 Sep 2. J Neurotrauma. 2020. PMID: 32808570 Free PMC article. Review.
-
Circulating Brain-Reactive Autoantibody Profiles in Military Breachers Exposed to Repetitive Occupational Blast.Int J Mol Sci. 2024 Dec 21;25(24):13683. doi: 10.3390/ijms252413683. Int J Mol Sci. 2024. PMID: 39769446 Free PMC article.
-
Early chronic systemic inflammation and associations with cognitive performance after moderate to severe TBI.Brain Behav Immun Health. 2020 Dec 15;11:100185. doi: 10.1016/j.bbih.2020.100185. eCollection 2021 Feb. Brain Behav Immun Health. 2020. PMID: 34589725 Free PMC article.
-
Neuroendocrine Disruptions Following Head Injury.Curr Neurol Neurosci Rep. 2023 May;23(5):213-224. doi: 10.1007/s11910-023-01263-5. Epub 2023 May 6. Curr Neurol Neurosci Rep. 2023. PMID: 37148402 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention, National Center for Injury Prevention and Control (2016). TBI: Get the facts. http://www.cdc.gov/traumaticbraininjury/get_the_facts.html (last accessed June7, 2016)
-
- Centers for Disease Control and Prevention, National Center for Injury Prevention and Control (2016). Rates of TBI-related emergency department visits, hospitalizations, and deaths by sex — United States, 2001–2010. http://www.cdc.gov/traumaticbraininjury/data/rates_bysex.html (last accessed June7, 2016)
-
- Zaloshnja E., Miller T., Langlois J.A., and Selassie A.W. (2008). Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehabil. 23, 394–400 - PubMed
-
- Kopczak A., Kilimann I., von Rosen F., Krewer C., Schneider H.J., Stalla G.K., and Schneider M. (2014). Screening for Hypopituitarism in 509 Patients with Traumatic Brain Injury or Subarachnoid Hemorrhage. J. Neurotrauma 31, 99–107 - PubMed
-
- Tanriverdi F., Schneider H.J., Aimaretti G., Masel B.E., Casanueva F.F., and Kelestimur F. (2015). Pituitary dysfunction after traumatic brain injury: a clinical and pathophysiological approach. Endocr. Rev. 36, 305–342 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

