Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro
- PMID: 32111238
- PMCID: PMC7049226
- DOI: 10.1186/s13287-020-01612-y
Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro
Abstract
Introduction: Mesenchymal stem cells (MSCs) exert immunomodulatory functions by inducing the development and differentiation of naive T cells into T cells with an anti-inflammatory regulatory T cell (Treg) phenotype. Our previous study showed that hepatocyte growth factor (HGF) secreted by MSCs had immunomodulatory effects in the context of lipopolysaccharide (LPS) stimulation. We hypothesized that HGF is a key factor in the MSC-mediated regulation of the T helper 17 (Th17) cell/regulatory T (Treg) cell balance.
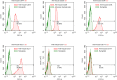
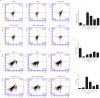
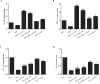
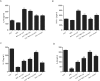
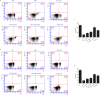
Methods: We investigated the effects of MSCs on the differentiation of CD4+ T cells and the functions of Th17/Treg cells in response to LPS stimulation by performing in vitro coculture experiments. MSCs were added to the upper chambers of cell culture inserts, and CD4+ T cells were plated in the lower chambers, followed by treatment with LPS or an anti-HGF antibody. Th17 (CD4+CD3+RORrt+) and Treg (CD4+CD25+Foxp3+) cell frequencies were analysed by flow cytometry, and the expression of Th17 cell- and Treg cell-related cytokines in the CD4+ T cells or culture medium was measured by quantitative PCR (qPCR) and enzyme-linked immunosorbent assay (ELISA), respectively. Neutrophil functions were determined by flow cytometry after a coculture with Th17/Treg cells.
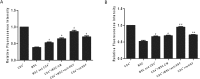
Results: The percentage of CD4+CD25+Foxp3+ cells was significantly increased in the CD4+ T cell population, while the percentage of CD4+CD3+RORrt+ cells was significantly decreased after MSC coculture. However, the MSC-induced effect was significantly inhibited by the anti-HGF antibody (p < 0.05). Furthermore, MSCs significantly inhibited the CD4+ T cell expression of IL-17 and IL-6 but increased the expression of IL-10 (p < 0.05 or p < 0.01); these effects were inhibited by the anti-HGF antibody (p < 0.05). In addition, CD4+ T cells cocultured with MSCs significantly inhibited neutrophil phagocytic and oxidative burst activities (p < 0.05 or p < 0.01); however, these MSC-induced effects were inhibited by the anti-HGF antibody (p < 0.05).
Conclusion: These data suggested that MSCs induced the conversion of fully differentiated Th17 cells into functional Treg cells and thereby modulated the Th17/Treg cell balance in the CD4+ T cell population, which was partly attributed to HGF secreted by the MSCs.
Keywords: Hepatocyte growth factor; Mesenchymal stem cells; Th17; Treg.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Adamzik M, Broll J, Steinmann J, Westendorf AM, et al. An increased alveolar CD4 + CD25 + Foxp3 + T-regulatory cell ratio in acute respiratory distress syndrome is associated with increased 30-day mortality. Intensive Care Med. 2013;39(10):1743–1751. doi: 10.1007/s00134-013-3036-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

