Role of Magnetic Resonance Imaging in the Evaluation of Breast Cancer Response to Neoadjuvant Chemotherapy
- PMID: 32111803
- PMCID: PMC7157867
- DOI: 10.21873/invivo.11857
Role of Magnetic Resonance Imaging in the Evaluation of Breast Cancer Response to Neoadjuvant Chemotherapy
Abstract
Background/aim: The aim of the study was to evaluate whether residual tumor assessment by magnetic resonance imaging (MRI) after neoadjuvant chemotherapy (NACT) is fundamental for a successive surgical strategy.
Patients and methods: We collected 55 MRIs performed after NACT.
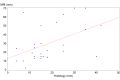
Results: Pathological response rate was 20%. MRI's sensitivity, specificity, PPV and NPV were 50%, 88%, 54% and 86%, respectively. We observed a high variability between the different subgroups, with high number of false positives in luminal A/B tumors. Triple negative and HER2+ tumors had almost the same specificity and sensitivity (81% and 50%). Nevertheless, in the HER2+ group, PPV was greater than that in the triple negative group (71% and 33% respectively) and the NPV of the triple negative group was greater than that of the HER2+ one (90% and 64%, respectively). Statistical analysis showed a weak but significant correlation between MRI and pathological assessment of residual tumor dimension.
Conclusion: The present study, confirms literature data about MRI accuracy in diagnosing HER2+ and triple negative tumors, but suggests caution in case of luminal tumors' evaluation.
Keywords: Magnetic resonance imaging; breast cancer subtypes; breast neoplasms; imaging complete response (iCR); neoadjuvant chemotherapy; pathological complete response (pCR).
Copyright© 2020, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors have no conflicts of interest to declare regarding this study.
Figures

References
-
- Hahn A, Schlotter CM, Rossmanith WG, Ulmer HU, Staiger HJ, Villena C. Neoadjuvant chemotherapy for breast cancer with weekly nab-paclitaxel followed by epirubicin and cyclophosphamide – results of a case series. In Vivo. 2014;28(2):235–241. - PubMed
-
- Kim HJ, Im YH, Han BK, Choi N, Lee J, Kim JH, Choi YL, Ahn JS, Nam SJ, Park YS, Choe YH, Ko YH, Yang JH. Accuracy of MRI for estimating residual tumor size after neoadjuvant chemotherapy in locally advanced breast cancer: relation to response patterns in MRI. Acta Oncol. 2007;26:996–1003. doi: 10.1080/02841860701373587. - DOI - PubMed
-
- Lobbes MBI, Smidt M, Tjan-Heijnen VCG, Van Goethem M, Schipper R, Beets-Tan RG, Wildberger JE. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging. 2013;4:163–175. doi: 10.1007/s13244-013-0219-y. - DOI - PMC - PubMed
-
- Park S, Yoon JH, Sohn J, Park HS, Moon HJ, Kim MJ, Kim EK, Kim SI, Park BW. Magnetic resonance imaging after completation of neoadjuvant chemotherapy can accurately discriminate between no residual carcinoma and residual ductal carcinoma in situ in patients with triple-negative breast cancer. PLoS One. 2016;11(2):e0149347. doi: 10.1371/journal.pone.0149347. - DOI - PMC - PubMed
-
- Lee SC, Grant E, Sheth P, Garcia AA, Bhushan Desai MB, Ji L, Groshen S, Hwang D, Yamashita M, Hovanessian-Larsen L. Accuracy of contrast-enhances ultrasound compared with magnetic resonance imaging in assessing the tumor response after neoadjuvant chemotherapy for breast cancer. J Ultrasound Med. 2017 doi: 10.7863/ultra.16.05060. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
