Pharmacological LRH-1/Nr5a2 inhibition limits pro-inflammatory cytokine production in macrophages and associated experimental hepatitis
- PMID: 32111818
- PMCID: PMC7048823
- DOI: 10.1038/s41419-020-2348-9
Pharmacological LRH-1/Nr5a2 inhibition limits pro-inflammatory cytokine production in macrophages and associated experimental hepatitis
Abstract
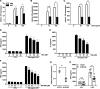
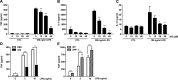
Liver receptor homolog-1 (LRH-1, Nr5a2) is an orphan nuclear receptor mainly expressed in tissues of endodermal origin, where its physiological role has been extensively studied. LRH-1 has been implicated in liver cell differentiation and proliferation, as well as glucose, lipid, and bile acid metabolism. In addition, increasing evidence highlights its role in immunoregulatory processes via glucocorticoid synthesis in the intestinal epithelium. Although the direct function of LRH-1 in immune cells is fairly elucidated, a role of LRH-1 in the regulation of macrophage differentiation has been recently reported. In this study, we aimed to investigate the role of LRH-1 in the regulation of pro-inflammatory cytokine production in macrophages. Our data demonstrate that pharmacological inhibition, along with LRH-1 knockdown, significantly reduced the lipopolysaccharide (LPS)-induced production of pro-inflammatory cytokines in the macrophage line RAW 264.7 cells, as well as in primary murine macrophages. This inhibitory effect was found to be independent of defects of LRH-1-regulated cell proliferation or toxic effects of the LRH-1 inhibitors. In contrast, LRH-1 inhibition reduced the mitochondrial ATP production and metabolism of macrophages through downregulation of the LRH-1 targets glucokinase and glutminase-2, and thus impairing the LPS-induced macrophage activation. Interestingly, in vivo pharmacological inhibition of LRH-1 also resulted in reduced tumor necrosis factor (TNF) production and associated decreased liver damage in a macrophage- and TNF-dependent mouse model of hepatitis. Noteworthy, despite hepatocytes expressing high levels of LRH-1, pharmacological inhibition of LRH-1 per se did not cause any obvious liver damage. Therefore, this study proposes LRH-1 as an emerging therapeutic target in the treatment of inflammatory disorders, especially where macrophages and cytokines critically decide the extent of inflammation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
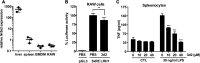



Similar articles
-
LRH-1/NR5A2 targets mitochondrial dynamics to reprogram type 1 diabetes macrophages and dendritic cells into an immune tolerance phenotype.Clin Transl Med. 2024 Dec;14(12):e70134. doi: 10.1002/ctm2.70134. Clin Transl Med. 2024. PMID: 39702941 Free PMC article.
-
The orphan nuclear receptor LRH-1/NR5a2 critically regulates T cell functions.Sci Adv. 2019 Jul 17;5(7):eaav9732. doi: 10.1126/sciadv.aav9732. eCollection 2019 Jul. Sci Adv. 2019. PMID: 31328159 Free PMC article.
-
The liver receptor homolog-1 (LRH-1) is expressed in human islets and protects {beta}-cells against stress-induced apoptosis.Hum Mol Genet. 2011 Jul 15;20(14):2823-33. doi: 10.1093/hmg/ddr193. Epub 2011 May 2. Hum Mol Genet. 2011. PMID: 21536586
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Nuclear-mitochondrial crosstalk: On the role of the nuclear receptor liver receptor homolog-1 (NR5A2) in the regulation of mitochondrial metabolism, cell survival, and cancer.IUBMB Life. 2021 Mar;73(3):592-610. doi: 10.1002/iub.2386. Epub 2020 Sep 15. IUBMB Life. 2021. PMID: 32931651 Review.
Cited by
-
Another One Bites the Gut: Nuclear Receptor LRH-1 in Intestinal Regeneration and Cancer.Cancers (Basel). 2021 Feb 20;13(4):896. doi: 10.3390/cancers13040896. Cancers (Basel). 2021. PMID: 33672730 Free PMC article. Review.
-
Mitogen-Activated Protein Kinase and Exploratory Nuclear Receptor Crosstalk in Cancer Immunotherapy.Int J Mol Sci. 2023 Sep 26;24(19):14546. doi: 10.3390/ijms241914546. Int J Mol Sci. 2023. PMID: 37833991 Free PMC article. Review.
-
LRH-1 activation alleviates diabetes-induced podocyte injury by promoting GLS2-mediated glutaminolysis.Cell Prolif. 2023 Nov;56(11):e13479. doi: 10.1111/cpr.13479. Epub 2023 Apr 13. Cell Prolif. 2023. PMID: 37057309 Free PMC article.
-
The versatility of liver X receptors in T cell homeostasis: Location, location, location!J Exp Med. 2021 Apr 5;218(4):e20202318. doi: 10.1084/jem.20202318. J Exp Med. 2021. PMID: 33475707 Free PMC article.
-
Remodeling the hepatic fibrotic microenvironment with emerging nanotherapeutics: a comprehensive review.J Nanobiotechnology. 2023 Apr 7;21(1):121. doi: 10.1186/s12951-023-01876-5. J Nanobiotechnology. 2023. PMID: 37029392 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

