Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis
- PMID: 32111836
- PMCID: PMC7048814
- DOI: 10.1038/s41467-020-14344-7
Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis
Abstract
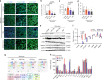
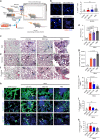
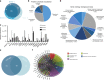
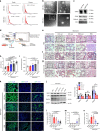
Idiopathic pulmonary fibrosis (IPF) is a fatal and incurable form of interstitial lung disease in which persistent injury results in scar tissue formation. As fibrosis thickens, the lung tissue loses the ability to facilitate gas exchange and provide cells with needed oxygen. Currently, IPF has few treatment options and no effective therapies, aside from lung transplant. Here we present a series of studies utilizing lung spheroid cell-secretome (LSC-Sec) and exosomes (LSC-Exo) by inhalation to treat different models of lung injury and fibrosis. Analysis reveals that LSC-Sec and LSC-Exo treatments could attenuate and resolve bleomycin- and silica-induced fibrosis by reestablishing normal alveolar structure and decreasing both collagen accumulation and myofibroblast proliferation. Additionally, LSC-Sec and LSC-Exo exhibit superior therapeutic benefits than their counterparts derived from mesenchymal stem cells in some measures. We showed that an inhalation treatment of secretome and exosome exhibited therapeutic potential for lung regeneration in two experimental models of pulmonary fibrosis.
Conflict of interest statement
K.C. is a co-founder and equity holder of BreStem Therapeutics. BreStem provided no funding to this study. The remaining authors declare no competing interests.
Figures


References
-
- Henry Eric, Cores Jhon, Hensley M. Taylor, Anthony Shirena, Vandergriff Adam, de Andrade James B.M., Allen Tyler, Caranasos Thomas G., Lobo Leonard J., Cheng Ke. Adult Lung Spheroid Cells Contain Progenitor Cells and Mediate Regeneration in Rodents With Bleomycin-Induced Pulmonary Fibrosis. STEM CELLS Translational Medicine. 2015;4(11):1265–1274. doi: 10.5966/sctm.2015-0062. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

