Neuronal contact guidance and YAP signaling on ultra-small nanogratings
- PMID: 32111918
- PMCID: PMC7048778
- DOI: 10.1038/s41598-020-60745-5
Neuronal contact guidance and YAP signaling on ultra-small nanogratings
Abstract
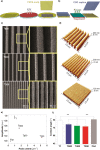
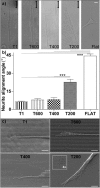
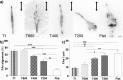
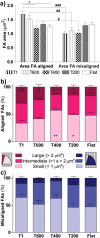
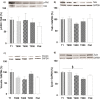
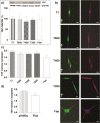
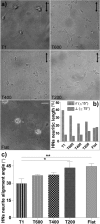
Contact interaction of neuronal cells with extracellular nanometric features can be exploited to investigate and modulate cellular responses. By exploiting nanogratings (NGs) with linewidth from 500 nm down to 100 nm, we here study neurite contact guidance along ultra-small directional topographies. The impact of NG lateral dimension on the neuronal morphotype, neurite alignment, focal adhesion (FA) development and YAP activation is investigated in nerve growth factor (NGF)-differentiating PC12 cells and in primary hippocampal neurons, by confocal and live-cell total internal reflection fluorescence (TIRF) microscopy, and at molecular level. We demonstrate that loss of neurite guidance occurs in NGs with periodicity below 400 nm and correlates with a loss of FA lateral constriction and spatial organization. We found that YAP intracellular localization is modulated by the presence of NGs, but it is not sensitive to their periodicity. Nocodazole, a drug that can increase cell contractility, is finally tested for rescuing neurite alignment showing mild ameliorative effects. Our results provide new indications for a rational design of biocompatible scaffolds for enhancing nerve-regeneration processes.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Neuronal differentiation on anisotropic substrates and the influence of nanotopographical noise on neurite contact guidance.Biomaterials. 2013 Aug;34(25):6027-36. doi: 10.1016/j.biomaterials.2013.04.039. Epub 2013 May 18. Biomaterials. 2013. PMID: 23694901
-
The effect of alternative neuronal differentiation pathways on PC12 cell adhesion and neurite alignment to nanogratings.Biomaterials. 2010 Mar;31(9):2565-73. doi: 10.1016/j.biomaterials.2009.12.010. Epub 2009 Dec 24. Biomaterials. 2010. PMID: 20035995
-
Interaction of SH-SY5Y cells with nanogratings during neuronal differentiation: comparison with primary neurons.Adv Healthc Mater. 2014 Apr;3(4):581-7. doi: 10.1002/adhm.201300216. Epub 2013 Oct 1. Adv Healthc Mater. 2014. PMID: 24115396
-
Activation of microtubule dynamics increases neuronal growth via the nerve growth factor (NGF)- and Gαs-mediated signaling pathways.J Biol Chem. 2015 Apr 17;290(16):10045-56. doi: 10.1074/jbc.M114.630632. Epub 2015 Feb 17. J Biol Chem. 2015. PMID: 25691569 Free PMC article.
-
Impaired Neurite Contact Guidance in Ubiquitin Ligase E3a (Ube3a)-Deficient Hippocampal Neurons on Nanostructured Substrates.Adv Healthc Mater. 2016 Apr 6;5(7):850-62. doi: 10.1002/adhm.201500815. Epub 2016 Feb 4. Adv Healthc Mater. 2016. PMID: 26845073
Cited by
-
Two-Photon Polymerization of 2.5D and 3D Microstructures Fostering a Ramified Resting Phenotype in Primary Microglia.Front Bioeng Biotechnol. 2022 Jul 22;10:926642. doi: 10.3389/fbioe.2022.926642. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35979173 Free PMC article.
-
PC12 differentiation to neuron cells activated by a low-level laser at 660 nm on UV pre-treated CR-39 scaffolds with parallel microchannels.Biomed Opt Express. 2024 Jul 12;15(8):4655-4674. doi: 10.1364/BOE.530876. eCollection 2024 Aug 1. Biomed Opt Express. 2024. PMID: 39347001 Free PMC article.
-
The influence of nanotopography on cell behaviour through interactions with the extracellular matrix - A review.Bioact Mater. 2021 Dec 21;15:145-159. doi: 10.1016/j.bioactmat.2021.11.024. eCollection 2022 Sep. Bioact Mater. 2021. PMID: 35386337 Free PMC article. Review.
-
The Combined Effects of Topography and Stiffness on Neuronal Differentiation and Maturation Using a Hydrogel Platform.Cells. 2023 Mar 18;12(6):934. doi: 10.3390/cells12060934. Cells. 2023. PMID: 36980275 Free PMC article.
-
Cellular and Subcellular Contact Guidance on Microfabricated Substrates.Front Bioeng Biotechnol. 2020 Oct 22;8:551505. doi: 10.3389/fbioe.2020.551505. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33195116 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

