Engineering a solid-state metalloprotein hydrogen evolution catalyst
- PMID: 32111964
- PMCID: PMC7048781
- DOI: 10.1038/s41598-020-60730-y
Engineering a solid-state metalloprotein hydrogen evolution catalyst
Abstract
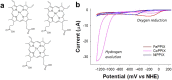
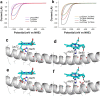
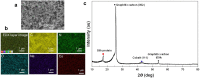
Hydrogen has the potential to play an important role in decarbonising our energy systems. Crucial to achieving this is the ability to produce clean sources of hydrogen using renewable energy sources. Currently platinum is commonly used as a hydrogen evolution catalyst, however, the scarcity and expense of platinum is driving the need to develop non-platinum-based catalysts. Here we report a protein-based hydrogen evolution catalyst based on a recombinant silk protein from honeybees and a metal macrocycle, cobalt protoporphyrin (CoPPIX). We enhanced the hydrogen evolution activity three fold compared to the unmodified silk protein by varying the coordinating ligands to the metal centre. Finally, to demonstrate the use of our biological catalyst, we built a proton exchange membrane (PEM) water electrolysis cell using CoPPIX-silk as the hydrogen evolution catalyst that is able to produce hydrogen with a 98% Faradaic efficiency. This represents an exciting advance towards allowing protein-based catalysts to be used in electrolysis cells.
Conflict of interest statement
The authors declare no competing interests.
Figures
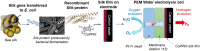

References
-
- Staffell I, et al. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019;12:463–491. doi: 10.1039/C8EE01157E. - DOI
-
- Voldsund M, Jordal K, Anantharaman R. Hydrogen production with CO2 capture. Int. J. Hydrogen Energy. 2016;41:4969–4992. doi: 10.1016/j.ijhydene.2016.01.009. - DOI
-
- Ju HK, Badwal S, Giddey S. A comprehensive review of carbon and hydrocarbon assisted water electrolysis for hydrogen production. Appl. Energy. 2018;231:502–533. doi: 10.1016/j.apenergy.2018.09.125. - DOI
-
- Badwal SPS, Giddey S, Munnings C. Emerging technologies, markets and commercialization of solid-electrolytic hydrogen production. Wiley Interdiscip. Rev. Energy Environ. 2018;7:1–19. doi: 10.1002/wene.286. - DOI
-
- Carmo M, Fritz DL, Mergel J, Stolten D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy. 2013;38:4901–4934. doi: 10.1016/j.ijhydene.2013.01.151. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

