Activation of Estrogen Receptor G Protein-Coupled Receptor 30 Enhances Cholesterol Cholelithogenesis in Female Mice
- PMID: 32112420
- PMCID: PMC8157628
- DOI: 10.1002/hep.31212
Activation of Estrogen Receptor G Protein-Coupled Receptor 30 Enhances Cholesterol Cholelithogenesis in Female Mice
Abstract
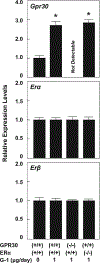
Background and aims: Estrogen is an important risk factor for cholesterol gallstone disease because women are twice as likely as men to form gallstones. The classical estrogen receptor α (ERα), but not ERβ, in the liver plays a critical role in the formation of estrogen-induced gallstones in female mice. The molecular mechanisms underlying the lithogenic effect of estrogen on gallstone formation have become more complicated with the identification of G protein-coupled receptor 30 (GPR30), an estrogen receptor.
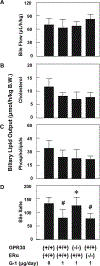
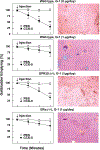
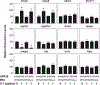
Approach and results: We investigated the biliary and gallstone phenotypes in ovariectomized female GPR30-/- , ERα-/- , and wild-type mice injected intramuscularly with the potent GPR30-selective agonist G-1 at 0 or 1 μg/day and fed a lithogenic diet for 8 weeks. The activation of GPR30 by G-1 enhanced cholelithogenesis by suppressing expression of cholesterol 7α-hydroxylase, the rate-limiting enzyme for the classical pathway of bile salt synthesis. These metabolic abnormalities led to an increase in biliary cholesterol concentrations in company with hepatic hyposecretion of biliary bile salts, thereby inducing cholesterol-supersaturated gallbladder bile and accelerating cholesterol crystallization. G-1 also impairs gallbladder emptying, leading to sluggish gallbladder motility and promoting the development of biliary sludge in the early stage of gallstone formation. The prevalence rates of gallstones were 80% in wild-type and ERα-/- mice treated with G-1 compared to 10% in wild-type mice receiving no G-1. However, no gallstones were formed in GPR30-/- mice treated with G-1.
Conclusions: GPR30 produces additional lithogenic actions, working independently of ERα, to increase susceptible to gallstone formation in female mice; both GPR30 and ERα are potential therapeutic targets for cholesterol gallstone disease, particularly in women and patients exposed to high levels of estrogen.
© 2020 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures


References
-
- Wang HH, Afdhal NH, Wang DQ. Estrogen receptor alpha, but not beta, plays a major role in 17beta-estradiol-induced murine cholesterol gallstones. Gastroenterology 2004;127:239–249. - PubMed
-
- Wang HH, Afdhal NH, Wang DQ. Overexpression of estrogen receptor alpha increases hepatic cholesterogenesis, leading to biliary hypersecretion in mice. J Lipid Res 2006;47:778–786. - PubMed
-
- Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 1997;45:607–617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

