Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling
- PMID: 32112426
- PMCID: PMC7703657
- DOI: 10.1111/all.14254
Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling
Abstract
Background: Short-chain fatty acids (SCFAs) are fermented dietary components that regulate immune responses, promote colonic health, and suppress mast cell-mediated diseases. However, the effects of SCFAs on human mast cell function, including the underlying mechanisms, remain unclear. Here, we investigated the effects of the SCFAs (acetate, propionate, and butyrate) on mast cell-mediated pathology and human mast cell activation, including the molecular mechanisms involved.
Method: Precision-cut lung slices (PCLS) of allergen-exposed guinea pigs were used to assess the effects of butyrate on allergic airway contraction. Human and mouse mast cells were co-cultured with SCFAs and assessed for degranulation after IgE- or non-IgE-mediated stimulation. The underlying mechanisms involved were investigated using knockout mice, small molecule inhibitors/agonists, and genomics assays.
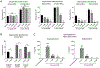
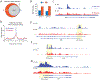
Results: Butyrate treatment inhibited allergen-induced histamine release and airway contraction in guinea pig PCLS. Propionate and butyrate, but not acetate, inhibited IgE- and non-IgE-mediated human or mouse mast cell degranulation in a concentration-dependent manner. Notably, these effects were independent of the stimulation of SCFA receptors GPR41, GPR43, or PPAR, but instead were associated with inhibition of histone deacetylases. Transcriptome analyses revealed butyrate-induced downregulation of the tyrosine kinases BTK, SYK, and LAT, critical transducers of FcεRI-mediated signals that are essential for mast cell activation. Epigenome analyses indicated that butyrate redistributed global histone acetylation in human mast cells, including significantly decreased acetylation at the BTK, SYK, and LAT promoter regions.
Conclusion: Known health benefits of SCFAs in allergic disease can, at least in part, be explained by epigenetic suppression of human mast cell activation.
Keywords: FcεRI signaling; butyrate; histone deacetylase; mast cells; short-chain fatty acids.
© 2020 EAACI and John Wiley and Sons A/S. Published by John Wiley and Sons Ltd.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflicts of interest.
Figures



References
-
- Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol 2014;121:91–119. - PubMed
-
- Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016;165:1332–1345. - PubMed
-
- Roduit C, Frei R, Ferstl R, Loeliger S, Westermann P, Rhyner C et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2018;40:833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

