Role of monocarboxylate transporters in regulating metabolic homeostasis in the outer retina: Insight gained from cell-specific Bsg deletion
- PMID: 32112484
- PMCID: PMC7849204
- DOI: 10.1096/fj.201902961R
Role of monocarboxylate transporters in regulating metabolic homeostasis in the outer retina: Insight gained from cell-specific Bsg deletion
Abstract
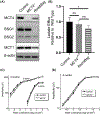
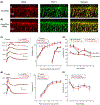
The neural retina metabolizes glucose through aerobic glycolysis generating large amounts of lactate. Lactate flux into and out of cells is regulated by proton-coupled monocarboxylate transporters (MCTs), which are encoded by members of the Slc16a family. MCT1, MCT3, and MCT4 are expressed in the retina and require association with the accessory protein basigin, encoded by Bsg, for maturation and trafficking to the plasma membrane. Bsg-/- mice have severely reduced electroretinograms (ERGs) and progressive photoreceptor degeneration, which is presumed to be driven by metabolic dysfunction resulting from loss of MCTs. To understand the basis of the Bsg-/- phenotype, we generated mice with conditional deletion of Bsg in rods (RodΔBsg), cones (Cone∆Bsg), or retinal pigment epithelial cells (RPEΔBsg). RodΔBsg mice showed a progressive loss of photoreceptors, while ConeΔBsg mice did not display a degenerative phenotype. The RPEΔBsg mice developed a distinct phenotype characterized by severely reduced ERG responses as early as 4 weeks of age. The loss of lactate transporters from the RPE most closely resembled the phenotype of the Bsg-/- mouse, suggesting that the regulation of lactate levels in the RPE and the subretinal space is essential for the viability and function of photoreceptors.
Keywords: RPE; basigin; lactate; monocarboxylate transporters; photoreceptors; retina.
© 2020 Federation of American Societies for Experimental Biology.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

