Intratumoral and s.c. injection of inactivated hemagglutinating virus of Japan envelope (GEN0101) in metastatic castration-resistant prostate cancer
- PMID: 32112659
- PMCID: PMC7226216
- DOI: 10.1111/cas.14366
Intratumoral and s.c. injection of inactivated hemagglutinating virus of Japan envelope (GEN0101) in metastatic castration-resistant prostate cancer
Abstract
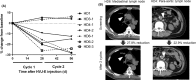
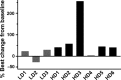
Inactivated hemagglutinating virus of Japan envelope (HVJ-E) has an antitumor effect and tumor immunity. We undertook an open-label, phase I, dose-escalation study in patients with castration-resistant prostate cancer (CRPC) to determine the safety and efficacy of intratumoral and s.c. injection of HVJ-E (GEN0101). Patients with CRPC, who were resistant to or unable to receive standard of care, were included. GEN0101 was injected directly into the prostate and s.c. in two 28-day treatment cycles. The primary end-points were to evaluate the safety and tolerability of GEN0101 and determine its recommended dose. The secondary end-points were to analyze the antitumor effect and tumor immunity. Three patients received 30 000 mNAU GEN0101 and 6 received 60 000 mNAU. There was no dose-limiting toxicity, and the recommended dose of GEN0101 was defined as 60 000 mNAU. Radiographically, 1 patient had stable disease and 2 had progressive disease in the low-dose group, whereas 5 patients had stable disease and 1 had progressive disease in the high-dose group. Three patients in the high-dose group showed reduction in lymph node metastasis. Prostate-specific antigen increase rates in the high-dose group were suppressed more than those in the low-dose group. Natural killer cell activity was enhanced in 2 patients of the low-dose group and in 5 patients in the high-dose group. In conclusion, intratumoral and s.c. injections of GEN0101 were well-tolerated and feasible to use. The study is registered with the UMIN Clinical Trials Registry (no. UMIN000017092).
Keywords: NK cell; Sendai virus; antitumor effect; castration-resistant prostate cancer; phase I clinical study.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Toshihiro Nakajima is the CEO of GenomIdea. Yasufumi Kaneda is a stock‐holder (0.3%) of GenomIdea. The other authors declare no conflicts of interest.
Figures



References
-
- Matsushima‐Miyagi T, Hatano K, Nomura M, et al. TRAIL and Noxa are selectively upregulated in prostate cancer cells downstream of the RIG‐I/MAVS signaling pathway by nonreplicating Sendai virus particles. Clin Cancer Res. 2012;18:6271‐6283. - PubMed
-
- Hatano K, Miyamoto Y, Nonomura N, Kaneda Y. Expression of gangliosides, GD1a, and sialyl paragloboside is regulated by NF‐B‐dependent transcriptional control of α2,3‐sialyltransferase I, II, and VI in human castration‐resistant prostate cancer cells. Int J Cancer. 2011;129:1838‐1847. - PubMed
-
- Kurooka M, Kaneda Y. Inactivated Sendai virus particles eradicate tumors by inducing immune responses through blocking regulatory T cells. Cancer Res. 2007;67:227‐236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

