Deferoxamine deconditioning increases neuronal vulnerability to hemoglobin
- PMID: 32112801
- PMCID: PMC7301423
- DOI: 10.1016/j.yexcr.2020.111926
Deferoxamine deconditioning increases neuronal vulnerability to hemoglobin
Abstract
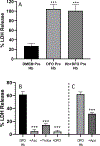
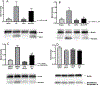
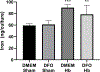
Concomitant treatment with deferoxamine (DFO) protects neural cells from iron and heme-mediated oxidative injury, but also disrupts cell responses to iron loading that may be protective. We hypothesized that DFO treatment and withdrawal would subsequently increase neuronal vulnerability to hemoglobin. Pretreatment with DFO followed by its washout increased neuronal loss after subsequent hemoglobin exposure by 3-4-fold compared with control vehicle-pretreated cultures. This was associated with reduced ferritin induction by hemoglobin; expression of heme oxygenase-1, which catalyzes iron release from heme, was not altered. Increased neuronal loss was prevented by exogenous apoferritin or by continuing DFO or antioxidants throughout the experimental course. Cell nonheme iron levels after hemoglobin treatment were similar in DFO-pretreated and control cultures. These results indicate that DFO deconditions neurons and subsequently increases their vulnerability to heme-mediated injury. Its net effect after CNS hemorrhage may be highly dependent on the timing and duration of its administration. Withdrawal of DFO while heme or iron levels remain elevated may be deleterious, and may negate any benefit of prior concomitant therapy.
Keywords: Deferoxamine; Intracerebral hemorrhage; Iron chelator; Neuronal death; Stroke; Subarachnoid hemorrhage.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures



References
-
- Bulters D, Gaastra B, Zolnourian A, Alexander S, Ren D, Blackburn SL, Borsody M, Dore S, Galea J, Iihara K, Nyquist P, Galea I, Haemoglobin scavenging in intracranial bleeding: biology and clinical implications, Nat. Rev. Neurol 14 (2018) 416–432. - PubMed
-
- Regan RF, Panter SS, Neurotoxicity of hemoglobin in cortical cell culture, Neurosci. Lett 153 (1993) 219–222. - PubMed
-
- Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G, Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage, J. Neurosurg 100 (2004) 672–678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

