Mycobacterium tuberculosis and myeloid-derived suppressor cells: Insights into caveolin rich lipid rafts
- PMID: 32113158
- PMCID: PMC7047144
- DOI: 10.1016/j.ebiom.2020.102670
Mycobacterium tuberculosis and myeloid-derived suppressor cells: Insights into caveolin rich lipid rafts
Abstract
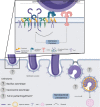
Mycobacterium tuberculosis (M.tb) is likely the most successful human pathogen, capable of evading protective host immune responses and driving metabolic changes to support its own survival and growth. Ineffective innate and adaptive immune responses inhibit effective clearance of the bacteria from the human host, resulting in the progression to active TB disease. Many regulatory mechanisms exist to prevent immunopathology, however, chronic infections result in the overproduction of regulatory myeloid cells, like myeloid-derived suppressor cells (MDSC), which actively suppress protective host T lymphocyte responses among other immunosuppressive mechanisms. The mechanisms of M.tb internalization by MDSC and the involvement of host-derived lipid acquisition, have not been fully elucidated. Targeted research aimed at investigating MDSC impact on phagocytic control of M.tb, would be advantageous to our collective anti-TB arsenal. In this review we propose a mechanism by which M.tb may be internalized by MDSC and survive via the manipulation of host-derived lipid sources.
Keywords: Caveolin; Internalization; Lipid metabolism; Mycobacterium tuberculosis; Myeloid-derived suppressor cells.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest All authors disclose that there are no conflicts of interest.
Figures
References
-
- Korb V.C., Chuturgoon A.A., Moodley D. Mycobacterium tuberculosis: manipulator of protective immunity. Int J Mol Sci [Internet] 2016;17(February 25 (3)) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4813124/ cited 2019 May 27]. Available from. - PMC - PubMed
-
- Goldberg M.F., Saini N.K., Porcelli S.A. Evasion of innate and adaptive immunity by Mycobacterium tuberculosis. Microbiol Spectr. 2014 Oct;2(5):1–21. - PubMed
-
- Brighenti S., Ordway D.J. Regulation of immunity to tuberculosis. Microbiol Spectr. 2016;4(6):1–24. - PubMed
-
- Mayer-Barber K.D., Barber D.L. Innate and adaptive cellular immune responses to Mycobacterium tuberculosis infection. Cold Spring Harb Perspect Med [Internet] 2015;5(December (12)) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4665043/ [cited 2019 May 27]. Available from. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

