The spinal microglial IL-10/β-endorphin pathway accounts for cinobufagin-induced mechanical antiallodynia in bone cancer pain following activation of α7-nicotinic acetylcholine receptors
- PMID: 32113469
- PMCID: PMC7049212
- DOI: 10.1186/s12974-019-1616-z
The spinal microglial IL-10/β-endorphin pathway accounts for cinobufagin-induced mechanical antiallodynia in bone cancer pain following activation of α7-nicotinic acetylcholine receptors
Abstract
Background: Cinobufagin is the major bufadienolide of Bufonis venenum (Chansu), which has been traditionally used for the treatment of chronic pain especially cancer pain. The current study aimed to evaluate its antinociceptive effects in bone cancer pain and explore the underlying mechanisms.
Methods: Rat bone cancer model was used in this study. The withdrawal threshold evoked by stimulation of the hindpaw was determined using a 2290 CE electrical von Frey hair. The β-endorphin and IL-10 levels were measured in the spinal cord and cultured primary microglia, astrocytes, and neurons.
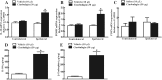
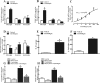
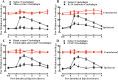
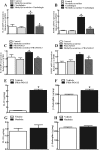
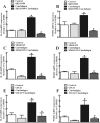
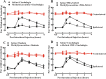
Results: Cinobufagin, given intrathecally, dose-dependently attenuated mechanical allodynia in bone cancer pain rats, with the projected Emax of 90% MPE and ED50 of 6.4 μg. Intrathecal cinobufagin also stimulated the gene and protein expression of IL-10 and β-endorphin (but not dynorphin A) in the spinal cords of bone cancer pain rats. In addition, treatment with cinobufagin in cultured primary spinal microglia but not astrocytes or neurons stimulated the mRNA and protein expression of IL-10 and β-endorphin, which was prevented by the pretreatment with the IL-10 antibody but not β-endorphin antiserum. Furthermore, spinal cinobufagin-induced mechanical antiallodynia was inhibited by the pretreatment with intrathecal injection of the microglial inhibitor minocycline, IL-10 antibody, β-endorphin antiserum and specific μ-opioid receptor antagonist CTAP. Lastly, cinobufagin- and the specific α-7 nicotinic acetylcholine receptor (α7-nAChR) agonist PHA-543613-induced microglial gene expression of IL-10/β-endorphin and mechanical antiallodynia in bone cancer pain were blocked by the pretreatment with the specific α7-nAChR antagonist methyllycaconitine.
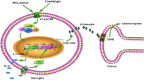
Conclusions: Our results illustrate that cinobufagin produces mechanical antiallodynia in bone cancer pain through spinal microglial expression of IL-10 and subsequent β-endorphin following activation of α7-nAChRs. Our results also highlight the broad significance of the recently uncovered spinal microglial IL-10/β-endorphin pathway in antinociception.
Keywords: Cinobufagin; IL-10/β-endorphin pathway; Microglia; α7-nicotinic acetylcholine receptor (α7-nAChR).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- AlSharari SD, Freitas K, Damaj MI. Functional role of alpha7 nicotinic receptor in chronic neuropathic and inflammatory pain: studies in transgenic mice. Biochem Pharmacol. 2013;86(8):1201–1207. - PubMed
-
- Baek SH, Kim C, Lee JH, Nam D, Lee J, Lee S-G, Ahn KS. Cinobufagin exerts anti-proliferative and pro-apoptotic effects through the modulation ROS-mediated MAPKs signaling pathway. Immunopharmacol Immunotoxicol. 2015;37(3):265–273. - PubMed
-
- Bick RJ, Poindexter BJ, Sweney RR, Dasgupta A. Effects of Chan Su, a traditional Chinese medicine, on the calcium transients of isolated cardiomyocytes: cardiotoxicity due to more than Na, K-ATPase blocking. Life Sci. 2002;72(6):699–709. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

