Lactate as a fulcrum of metabolism
- PMID: 32113910
- PMCID: PMC7284908
- DOI: 10.1016/j.redox.2020.101454
Lactate as a fulcrum of metabolism
Abstract
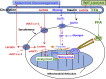
Mistakenly thought to be the consequence of oxygen lack in contracting skeletal muscle we now know that the L-enantiomer of the lactate anion is formed under fully aerobic conditions and is utilized continuously in diverse cells, tissues, organs and at the whole-body level. By shuttling between producer (driver) and consumer (recipient) cells lactate fulfills at least three purposes: 1] a major energy source for mitochondrial respiration; 2] the major gluconeogenic precursor; and 3] a signaling molecule. Working by mass action, cell redox regulation, allosteric binding, and reprogramming of chromatin by lactylation of lysine residues on histones, lactate has major influences in energy substrate partitioning. The physiological range of tissue [lactate] is 0.5-20 mM and the cellular Lactate/Pyruvate ratio (L/P) can range from 10 to >500; these changes during exercise and other stress-strain responses dwarf other metabolic signals in magnitude and span. Hence, lactate dynamics have rapid and major short- and long-term effects on cell redox and other control systems. By inhibiting lipolysis in adipose via HCAR-1, and muscle mitochondrial fatty acid uptake via malonyl-CoA and CPT1, lactate controls energy substrate partitioning. Repeated lactate exposure from regular exercise results in major effects on the expression of regulatory enzymes of glycolysis and mitochondrial respiration. Lactate is the fulcrum of metabolic regulation in vivo.
Keywords: Aerobic; Anaerobic; Cell-cell signaling; Energy substrate partitioning; Exercise; Gluconeogenesis; Glycolysis; HCAR1; Histone lactylation; Mitochondrial biogenesis; Oxidative metabolism; PGC-1α; PPAR-γ; SIRT activation; TGFβ.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The author has no competing interests to declare.
Figures





References
-
- Ahmed K., Tunaru S., Tang C., Muller M., Gille A., Sassmann A., Hanson J., Offermanns S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metabol. 2010;11:311–319. - PubMed
-
- Barros L.F. Metabolic signaling by lactate in the brain. Trends Neurosci. 2013;36:396–404. - PubMed
-
- Bayod S., Del Valle J., Lalanza J.F., Sanchez-Roige S., de Luxan-Delgado B., Coto-Montes A., Canudas A.M., Camins A., Escorihuela R.M., Pallas M. Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Exp. Gerontol. 2012;47:925–935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

