Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests
- PMID: 32114193
- PMCID: PMC7129110
- DOI: 10.1016/j.ijid.2020.02.050
Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests
Abstract
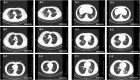
An ongoing outbreak of severe respiratory pneumonia associated with the 2019 novel coronavirus has recently emerged in China. Here we report the epidemiological, clinical, laboratory and radiological characteristics of 19 suspect cases. We compared the positive ratio of 2019-nCoV nucleic acid amplification test results from different samples including oropharyngeal swab, blood, urine and stool with 3 different fluorescent RT-PCR kits. Nine out of the 19 patients had 2019-nCoV infection detected using oropharyngeal swab samples, and the virus nucleic acid was also detected in eight of these nine patients using stool samples. None of positive results was identified in the blood and urine samples. These three different kits got the same result for each sample and the positive ratio of nucleic acid detection for 2019-nCoV was only 47.4% in the suspect patients. Therefore, it is possible that infected patients have been missed by using nucleic acid detection only. It might be better to make a diagnosis combining the computed tomography scans and nucleic acid detection.
Keywords: 2019 Novel coronavirus pneumonia; Clinical diagnosis; Nucleic acid amplification test.
Copyright © 2020 University of Electronic Science and Technology of China, Chengdu, China. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. - PubMed
-
- To K.K.W., Yip C.C.Y., Lai C.Y.W., Wong C.K.H., Ho D.T.Y., Pang P.K.P. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect. 2019;25(3):372–378. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

