Receptor tyrosine kinase activation: From the ligand perspective
- PMID: 32114309
- PMCID: PMC7813211
- DOI: 10.1016/j.ceb.2020.01.016
Receptor tyrosine kinase activation: From the ligand perspective
Abstract
Receptor tyrosine kinases (RTKs) are single-span transmembrane receptors in which relatively conserved intracellular kinase domains are coupled to divergent extracellular modules. The extracellular domains initiate receptor signaling upon binding to either soluble or membrane-embedded ligands. The diversity of extracellular domain structures allows for coupling of many unique signaling inputs to intracellular tyrosine phosphorylation. The combinatorial power of this receptor system is further increased by the fact that multiple ligands can typically interact with the same receptor. Such ligands often act as biased agonists and initiate distinct signaling responses via activation of the same receptor. Mechanisms behind such biased agonism are largely unknown for RTKs, especially at the level of receptor-ligand complex structure. Using recent progress in understanding the structures of active RTK signaling units, we discuss selected mechanisms by which ligands couple receptor activation to distinct signaling outputs.
Keywords: Biased agonism; Growth factor; Ligand; Receptor tyrosine kinase; Signaling.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures

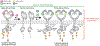
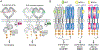

References
-
- Falls D: Neuregulins: functions, forms, and signaling strategies. Experimental Cell Research 2003, 284:14–30. - PubMed
-
- Flanagan JG, Gale NW, Hunter T, Pasquale EB, TessierLavigne M: Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell 1997, 90:403–404. - PubMed
-
- Pasquale EB: Eph receptor signalling casts a wide net on cell behaviour. Nature Reviews Molecular Cell Biology 2005, 6:462–475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

