Medroxyprogesterone acetate plus metformin for fertility-sparing treatment of atypical endometrial hyperplasia and endometrial carcinoma: trial protocol for a prospective, randomised, open, blinded-endpoint design, dose-response trial (FELICIA trial)
- PMID: 32114477
- PMCID: PMC7050341
- DOI: 10.1136/bmjopen-2019-035416
Medroxyprogesterone acetate plus metformin for fertility-sparing treatment of atypical endometrial hyperplasia and endometrial carcinoma: trial protocol for a prospective, randomised, open, blinded-endpoint design, dose-response trial (FELICIA trial)
Abstract
Introduction: Progestin therapy is the only fertility-sparing treatment option for patients with atypical endometrial hyperplasia (AEH) and endometrial cancer (EC). However, the results of three meta-analyses revealed a high remission rate, as well as an association with a high rate of relapse. We previously conducted a phase II of medroxyprogesterone acetate (MPA) plus metformin as a fertility-sparing treatment for AEH and EC patients, and reported that metformin inhibited disease relapse after remission.
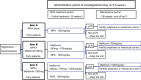
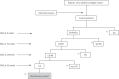
Methods and analysis: A randomised, open, blinded-endpoint design phase IIb dose response trial was planned to commence in July 2019. The trial aims to identify the appropriate dose of metformin to be combined with MPA therapy for fertility-sparing treatment of patients with AEH and EC. The primary endpoint of the trial is the 3-year relapse-free survival (RFS) rate. The secondary endpoints are RFS rate, the overall rate of response to MPA therapy, the conception rate after treatment, the outcome of pregnancy, toxicity evaluation and changes in insulin resistance and body mass index. A total of 120 patients will be enrolled from 15 Japanese institutions within a 2.5-year period and followed up for at least 3 years.
Ethics and dissemination: The protocol was approved by the institutional review board at Chiba University Hospital and boards at 14 other institutions. The trial will be conducted according to the principles of the World Medical Association's Declaration of Helsinki and in accordance with Good Clinical Practice (GCP) standards. The trial findings will be published in a peer-reviewed journal.
Trial registration number: Japan Registry of Clinical Trials (jRCT2031190065).
Keywords: adult oncology; gynaecological oncology.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: HH has received personal fees from Torii Yakuhin, outside the submitted work.
Figures
References
-
- National Comprehensive Cancer Network Uterine neoplasms: NCCN clinical practice guidelines in oncology (NCCN Guidelines®). Available: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf [Accessed 11 Feb 2019].
-
- Rodolakis A, Biliatis I, Morice P, et al. European Society of gynecological oncology Task force for fertility preservation: clinical recommendations for fertility-sparing management in young endometrial cancer patients. Int J Gynecol Cancer 2015;25:1258–65. 10.1097/IGC.0000000000000493 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
