CORE-Kids: a protocol for the development of a core outcome set for childhood fractures
- PMID: 32114480
- PMCID: PMC7050303
- DOI: 10.1136/bmjopen-2019-036224
CORE-Kids: a protocol for the development of a core outcome set for childhood fractures
Abstract
Introduction: Limb fractures in children are common yet there are few trials that compare treatments for these injuries. There is significant heterogeneity in the outcomes reported in the paediatric orthopaedic literature, which limits the ability to compare study results and draw firm conclusions. The aim of the CORE-Kids Study is to develop a core outcome set for use in research studies of childhood limb fractures. A core outcome set will provide a minimum set of outcomes to be measured in all trials to minimise the heterogeneity of outcomes reported and minimise reporting bias. A core outcome set ensures that outcomes are reported that are relevant to families as well as clinicians. The core outcome set will include additional upper and lower limb modules.
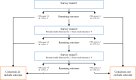
Methods: The development of the core outcome set will require four phases to evaluate:What are the outcomes that are relevant to professionals?What are the outcomes that are relevant to families?What are the most important of these outcomes?Which outcomes should be included in the core outcome set?This will be completed through a systematic review of trials to identify the outcomes domains that are relevant to trialists. A series of semi-structured interviews will be completed with families to identify the outcome domains that are relevant to families. These outcome domains will be used in a three-round Delphi Study to analyse the importance of these outcome domains to a range of stakeholders including parents, clinicians and researchers. Following this, the core outcome set will be decided at a consensus meeting.
Ethics and dissemination: Ethical approval has been awarded HRA/REC IRAS number 262503. Date of approval 06/08/2019. Dissemination will be through scientific literature and international societies.
Trial registration: Core Outcome Measures in Effectiveness Trials Initiative, registration number: 1274. Date of registration 13/12/2018.
Prospero registration number: CRD42018106605.
Keywords: paediatric orthopaedics; qualitative research; trauma management.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Peden M, Oyegbite K, Ozanne-Smith J, et al. World report on child injury prevention. Geneva, 2008. - PubMed
-
- European association for injury prevention and safety promotion. injuries in the European Union. Amsterdam 2014.
-
- Abraham A, Kumar S, Chaudhry S, et al. Surgical interventions for diaphyseal fractures of the radius and ulna in children Cochrane database of systematic reviews. Chichester, UK: John Wiley & Sons, Ltd, 2011. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
