Effect of seminal redox status on lipid peroxidation, apoptosis and DNA fragmentation in spermatozoa of infertile Saudi males
- PMID: 32114595
- PMCID: PMC7841563
- DOI: 10.15537/smj.2020.3.24975
Effect of seminal redox status on lipid peroxidation, apoptosis and DNA fragmentation in spermatozoa of infertile Saudi males
Abstract
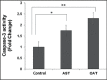
Objectives: To assess the effect of seminal redox status on lipid peroxidation (LPO), apoptosis and integrity of sperm DNA in infertile males. Methods: In this case-control study, the total antioxidant status (TAS) and reactive oxygen species (ROS) levels were analyzed within the seminal plasma of fertile normozoospermic, n=40 and infertile (asthenozoospermic, n=30; oligoasthenoteratozoospermic, n=30) males. Additionally, the level of 4-hydroxynonenal (4-HNE), DNA fragmentation, and caspase-3 activity were estimated in the spermatozoa.
Results: Significantly (p less than 0.001) increased seminal ROS level with decreased TAS scores was observed in the infertile groups compared to normozoospermics. The infertile males showed marked elevated (p less than 0.001) levels of 4-HNE, DNA fragmentation and caspase-3 activity compared to normozoospermics, which was positively correlated to increased seminal ROS levels and negatively to the TAS score in the studied groups. Seminal ROS level was significantly inverse correlated to the semen parameters. Additionally, a strong negative correlation between DNA fragmentation, LPO, caspase-3activity and seminal parameters were observed. Conclusion: Seminal oxidative stress is a potential risk factor for LPO, DNA damage, and apoptosis in spermatozoa, which can affect semen quality and male fertility. Thus, in addition to conventional seminological parameters, measurement of seminal oxidative stress and sperm DNA integrity may also be employed to investigate the functional integrity of spermatozoa at the molecular level.
Figures



Similar articles
-
Effects of reduced seminal enzymatic antioxidants on sperm DNA fragmentation and semen quality of Tunisian infertile men.J Assist Reprod Genet. 2017 Mar;34(3):373-381. doi: 10.1007/s10815-013-9936-x. Epub 2013 Jan 25. J Assist Reprod Genet. 2017. PMID: 23354588 Free PMC article.
-
Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality.Reprod Biomed Online. 2012 Sep;25(3):300-6. doi: 10.1016/j.rbmo.2012.05.011. Epub 2012 May 30. Reprod Biomed Online. 2012. PMID: 22818093
-
Increased Sperm DNA Fragmentation in Infertile Men with Varicocele: Relationship with Apoptosis, Seminal Oxidative Stress, and Spermatic Parameters.Reprod Sci. 2021 Mar;28(3):909-919. doi: 10.1007/s43032-020-00311-6. Epub 2020 Sep 9. Reprod Sci. 2021. PMID: 32909188
-
Withania somnifera improves semen quality by combating oxidative stress and cell death and improving essential metal concentrations.Reprod Biomed Online. 2011 May;22(5):421-7. doi: 10.1016/j.rbmo.2011.01.010. Epub 2011 Mar 8. Reprod Biomed Online. 2011. PMID: 21388887 Review.
-
[The redox system in human semen and peroxidative damage of spermatozoa].Postepy Hig Med Dosw (Online). 2005;59:523-34. Postepy Hig Med Dosw (Online). 2005. PMID: 16407791 Review. Polish.
Cited by
-
Ameliorating effect of Mucuna pruriens seed extract on sodium arsenite-induced testicular toxicity and hepato-renal histopathology in rats.Vet World. 2023 Jan;16(1):82-93. doi: 10.14202/vetworld.2023.82-93. Epub 2023 Jan 11. Vet World. 2023. PMID: 36855363 Free PMC article.
-
Antioxidants Present in Reproductive Tract Fluids and Their Relevance for Fertility.Antioxidants (Basel). 2021 Sep 9;10(9):1441. doi: 10.3390/antiox10091441. Antioxidants (Basel). 2021. PMID: 34573073 Free PMC article. Review.
-
Extend the Survival of Human Sperm In Vitro in Non-Freezing Conditions: Damage Mechanisms, Preservation Technologies, and Clinical Applications.Cells. 2022 Sep 12;11(18):2845. doi: 10.3390/cells11182845. Cells. 2022. PMID: 36139420 Free PMC article. Review.
-
Vitamin D Levels and Risk of Male Factor Infertility: A Mendelian Randomization Study.World J Mens Health. 2023 Jul;41(3):640-648. doi: 10.5534/wjmh.220109. Epub 2023 Jan 1. World J Mens Health. 2023. PMID: 36593707 Free PMC article.
-
From Hypoxia to Oxidative Stress: Antioxidants' Role to Reduce Male Reproductive Damage.Reprod Sci. 2025 Feb;32(2):261-277. doi: 10.1007/s43032-024-01746-x. Epub 2024 Nov 18. Reprod Sci. 2025. PMID: 39557807 Review.
References
-
- Inhorn MC, Patrizio P. Infertility around the globe:new thinking on the gender, and reproductive technology and global movement in 21st centuary. Hum Reprod Update. 2015;21:411–426. - PubMed
-
- Velu A, Prasad G. Epidemiologic aspects of male infertility. Int J Reprod Contracept Obstet Gynecol. 2017;6:3362–3365.
-
- AlEnezi H, Isa AM, Abu-Rafea B, Madbouly K, Binsaleh S. Pattern of semen fluid abnormalities in male partners of infertile couples in Riyadh, Saudi Arabia. Can J Urol. 2014;21:7322–7325. - PubMed
-
- Binsaleh S, Al-Qahtani R, Madbouly K, Isa AM, Abu-Rafea B. Evaluation of sperm DNA damage in men from infertile Saudi couples. J Reprod Med. 2015;60:135–140. - PubMed
-
- Al-Turki HA. Prevalence of primary and secondary infertility from tertiary center in eastern Saudi Arabia. Middle East Fertil Soc J. 2015;20:237–240.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

