A chimeric yellow fever-Zika virus vaccine candidate fully protects against yellow fever virus infection in mice
- PMID: 32116148
- PMCID: PMC7067203
- DOI: 10.1080/22221751.2020.1730709
A chimeric yellow fever-Zika virus vaccine candidate fully protects against yellow fever virus infection in mice
Abstract
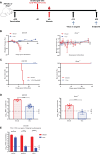
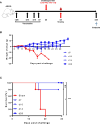
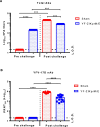
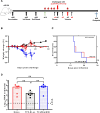
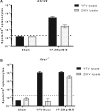
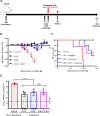
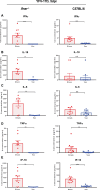
The recent Zika virus (ZIKV) epidemic in the Americas, followed by the yellow fever virus (YFV) outbreaks in Angola and Brazil highlight the urgent need for safe and efficient vaccines against the ZIKV as well as much greater production capacity for the YFV-17D vaccine. Given that the ZIKV and the YFV are largely prevalent in the same geographical areas, vaccines that would provide dual protection against both pathogens may obviously offer a significant benefit. We have recently engineered a chimeric vaccine candidate (YF-ZIKprM/E) by swapping the sequences encoding the YFV-17D surface glycoproteins prM/E by the corresponding sequences of the ZIKV. A single vaccine dose of YF-ZIKprM/E conferred complete protection against a lethal challenge with wild-type ZIKV strains. Surprisingly, this vaccine candidate also efficiently protected against lethal YFV challenge in various mouse models. We demonstrate that CD8+ but not CD4+ T cells, nor ZIKV neutralizing antibodies are required to confer protection against YFV. The chimeric YF-ZIKprM/E vaccine may thus be considered as a dual vaccine candidate efficiently protecting mice against both the ZIKV and the YFV, and this following a single dose immunization. Our finding may be particularly important in the rational design of vaccination strategies against flaviviruses, in particular in areas where YFV and ZIKV co-circulate.
Keywords: CD8+ T cells; YFV-17D; chimeric flavivirus vaccine; live-attenuated vaccines; non-neutralizing antibodies.
Conflict of interest statement
D.B.K, N.M, J.N. and K.D have filed a patent application claiming the discovery and use of chimeric yellow fever-Zika virus vaccines.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
