ECoG Beta Suppression and Modulation During Finger Extension and Flexion
- PMID: 32116497
- PMCID: PMC7031656
- DOI: 10.3389/fnins.2020.00035
ECoG Beta Suppression and Modulation During Finger Extension and Flexion
Abstract
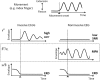
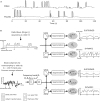
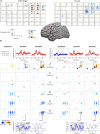
Neural oscillations originate predominantly from interacting cortical neurons and consequently reflect aspects of cortical information processing. However, their functional role is not yet fully understood and their interpretation is debatable. Amplitude modulations (AMs) in alpha (8-12 Hz), beta (13-30 Hz), and high gamma (70-150 Hz) band in invasive electrocorticogram (ECoG) and non-invasive electroencephalogram (EEG) signals change with behavior. Alpha and beta band AMs are typically suppressed (desynchronized) during motor behavior, while high gamma AMs highly correlate with the behavior. These two phenomena are successfully used for functional brain mapping and brain-computer interface (BCI) applications. Recent research found movement-phase related AMs (MPA) also in high beta/low gamma (24-40 Hz) EEG rhythms. These MPAs were found by separating the suppressed AMs into sustained and dynamic components. Sustained AM components are those with frequencies that are lower than the motor behavior. Dynamic components those with frequencies higher than the behavior. In this paper, we study ECoG beta/low gamma band (12-30 Hz/30-42 Hz) AM during repetitive finger movements addressing the question whether or not MPAs can be found in ECoG beta band. Indeed, MPA in the 12-18 Hz and 18-24 Hz band were found. This additional information may lead to further improvements in ECoG-based prediction and reconstruction of motor behavior by combining high gamma AM and beta band MPA.
Keywords: beta band; brain-computer interface; electrocorticogram; high gamma; movement-phase related amplitude modulation.
Copyright © 2020 Unterweger, Seeber, Zanos, Ojemann and Scherer.
Figures


References
LinkOut - more resources
Full Text Sources

