Axonal Endoplasmic Reticulum Dynamics and Its Roles in Neurodegeneration
- PMID: 32116502
- PMCID: PMC7025499
- DOI: 10.3389/fnins.2020.00048
Axonal Endoplasmic Reticulum Dynamics and Its Roles in Neurodegeneration
Abstract
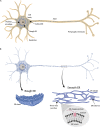
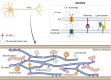
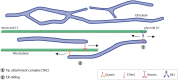
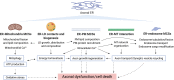
The physical continuity of axons over long cellular distances poses challenges for their maintenance. One organelle that faces this challenge is endoplasmic reticulum (ER); unlike other intracellular organelles, this forms a physically continuous network throughout the cell, with a single membrane and a single lumen. In axons, ER is mainly smooth, forming a tubular network with occasional sheets or cisternae and low amounts of rough ER. It has many potential roles: lipid biosynthesis, glucose homeostasis, a Ca2+ store, protein export, and contacting and regulating other organelles. This tubular network structure is determined by ER-shaping proteins, mutations in some of which are causative for neurodegenerative disorders such as hereditary spastic paraplegia (HSP). While axonal ER shares many features with the tubular ER network in other contexts, these features must be adapted to the long and narrow dimensions of axons. ER appears to be physically continuous throughout axons, over distances that are enormous on a subcellular scale. It is therefore a potential channel for long-distance or regional communication within neurons, independent of action potentials or physical transport of cargos, but involving its physiological roles such as Ca2+ or organelle homeostasis. Despite its apparent stability, axonal ER is highly dynamic, showing features like anterograde and retrograde transport, potentially reflecting continuous fusion and breakage of the network. Here we discuss the transport processes that must contribute to this dynamic behavior of ER. We also discuss the model that these processes underpin a homeostatic process that ensures both enough ER to maintain continuity of the network and repair breaks in it, but not too much ER that might disrupt local cellular physiology. Finally, we discuss how failure of ER organization in axons could lead to axon degenerative diseases, and how a requirement for ER continuity could make distal axons most susceptible to degeneration in conditions that disrupt ER continuity.
Keywords: axonal transport; calcium stores; endoplasmic reticulum; hereditary spastic paraplegia; neurodegeneration; organelle contact sites; smooth ER.
Copyright © 2020 Öztürk, O’Kane and Pérez-Moreno.
Figures
Similar articles
-
Modeling of axonal endoplasmic reticulum network by spastic paraplegia proteins.Elife. 2017 Jul 25;6:e23882. doi: 10.7554/eLife.23882. Elife. 2017. PMID: 28742022 Free PMC article.
-
Neuronal endoplasmic reticulum architecture and roles in axonal physiology.Mol Cell Neurosci. 2023 Jun;125:103822. doi: 10.1016/j.mcn.2023.103822. Epub 2023 Feb 11. Mol Cell Neurosci. 2023. PMID: 36781033 Review.
-
Liver X receptor-agonist treatment rescues degeneration in a Drosophila model of hereditary spastic paraplegia.Acta Neuropathol Commun. 2022 Mar 28;10(1):40. doi: 10.1186/s40478-022-01343-6. Acta Neuropathol Commun. 2022. PMID: 35346366 Free PMC article.
-
NeurodegenERation: The Central Role for ER Contacts in Neuronal Function and Axonopathy, Lessons From Hereditary Spastic Paraplegias and Related Diseases.Front Neurosci. 2019 Oct 11;13:1051. doi: 10.3389/fnins.2019.01051. eCollection 2019. Front Neurosci. 2019. PMID: 31680803 Free PMC article. Review.
-
The interconnection of endoplasmic reticulum and microtubule and its implication in Hereditary Spastic Paraplegia.Comput Struct Biotechnol J. 2023 Feb 20;21:1670-1677. doi: 10.1016/j.csbj.2023.02.025. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 36860342 Free PMC article. Review.
Cited by
-
Pathological characteristics of axons and alterations of proteomic and lipidomic profiles in midbrain dopaminergic neurodegeneration induced by WDR45-deficiency.Mol Neurodegener. 2024 Aug 26;19(1):62. doi: 10.1186/s13024-024-00746-4. Mol Neurodegener. 2024. PMID: 39183331 Free PMC article.
-
Hereditary Spastic Paraplegia and Future Therapeutic Directions: Beneficial Effects of Small Compounds Acting on Cellular Stress.Front Neurosci. 2021 May 6;15:660714. doi: 10.3389/fnins.2021.660714. eCollection 2021. Front Neurosci. 2021. PMID: 34025345 Free PMC article. Review.
-
Dynamic changes in endoplasmic reticulum morphology and its contact with the plasma membrane in motor neurons in response to nerve injury.Cell Tissue Res. 2024 Apr;396(1):71-84. doi: 10.1007/s00441-024-03858-x. Epub 2024 Feb 5. Cell Tissue Res. 2024. PMID: 38311679 Free PMC article.
-
The Endoplasmic Reticulum and Its Contacts: Emerging Roles in Axon Development, Neurotransmission, and Degeneration.Neuroscientist. 2024 Oct;30(5):545-559. doi: 10.1177/10738584231162810. Epub 2023 Mar 24. Neuroscientist. 2024. PMID: 36960757 Free PMC article. Review.
-
Regulation of ER Composition and Extent, and Putative Action in Protein Networks by ER/NE Protein TMEM147.Int J Mol Sci. 2021 Sep 23;22(19):10231. doi: 10.3390/ijms221910231. Int J Mol Sci. 2021. PMID: 34638576 Free PMC article.
References
-
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. (2002). “The endoplasmic reticulum,” in Molecular Biology of the Cell, ed. Alberts B., (New York, NY: Garland Science; ).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

