Cortical and Striatal Circuits in Huntington's Disease
- PMID: 32116525
- PMCID: PMC7025546
- DOI: 10.3389/fnins.2020.00082
Cortical and Striatal Circuits in Huntington's Disease
Abstract
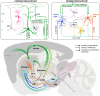
Huntington's disease (HD) is a hereditary neurodegenerative disorder that typically manifests in midlife with motor, cognitive, and/or psychiatric symptoms. The disease is caused by a CAG triplet expansion in exon 1 of the huntingtin gene and leads to a severe neurodegeneration in the striatum and cortex. Classical electrophysiological studies in genetic HD mouse models provided important insights into the disbalance of excitatory, inhibitory and neuromodulatory inputs, as well as progressive disconnection between the cortex and striatum. However, the involvement of local cortical and striatal microcircuits still remains largely unexplored. Here we review the progress in understanding HD-related impairments in the cortical and basal ganglia circuits, and outline new opportunities that have opened with the development of modern circuit analysis methods. In particular, in vivo imaging studies in mouse HD models have demonstrated early structural and functional disturbances within the cortical network, and optogenetic manipulations of striatal cell types have started uncovering the causal roles of certain neuronal populations in disease pathogenesis. In addition, the important contribution of astrocytes to HD-related circuit defects has recently been recognized. In parallel, unbiased systems biology studies are providing insights into the possible molecular underpinnings of these functional defects at the level of synaptic signaling and neurotransmitter metabolism. With these approaches, we can now reach a deeper understanding of circuit-based HD mechanisms, which will be crucial for the development of effective and targeted therapeutic strategies.
Keywords: Huntington’s disease; basal ganglia; cortex; genetic mouse models; in vivo calcium imaging; neural circuits; optogenetics.
Copyright © 2020 Blumenstock and Dudanova.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

