Expanding Clinical Presentations Due to Variations in THOC2 mRNA Nuclear Export Factor
- PMID: 32116545
- PMCID: PMC7026477
- DOI: 10.3389/fnmol.2020.00012
Expanding Clinical Presentations Due to Variations in THOC2 mRNA Nuclear Export Factor
Abstract
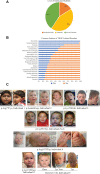
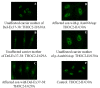
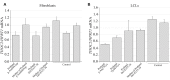
Multiple TREX mRNA export complex subunits (e.g., THOC1, THOC2, THOC5, THOC6, THOC7) have now been implicated in neurodevelopmental disorders (NDDs), neurodegeneration and cancer. We previously implicated missense and splicing-defective THOC2 variants in NDDs and a broad range of other clinical features. Here we report 10 individuals from nine families with rare missense THOC2 variants including the first case of a recurrent variant (p.Arg77Cys), and an additional individual with an intragenic THOC2 microdeletion (Del-Ex37-38). Ex vivo missense variant testing and patient-derived cell line data from current and published studies show 9 of the 14 missense THOC2 variants result in reduced protein stability. The splicing-defective and deletion variants result in a loss of small regions of the C-terminal THOC2 RNA binding domain (RBD). Interestingly, reduced stability of THOC2 variant proteins has a flow-on effect on the stability of the multi-protein TREX complex; specifically on the other NDD-associated THOC subunits. Our current, expanded cohort refines the core phenotype of THOC2 NDDs to language disorder and/or ID, with a variable severity, and disorders of growth. A subset of affected individuals' has severe-profound ID, persistent hypotonia and respiratory abnormalities. Further investigations to elucidate the pathophysiological basis for this severe phenotype are warranted.
Keywords: THOC2; intellectual disability; mRNA export; microdeletion; neurodevelopmental disorders.
Copyright © 2020 Kumar, Palmer, Gardner, Carroll, Banka, Abdelhadi, Donnai, Elgersma, Curry, Gardham, Suri, Malla, Brady, Tarnopolsky, Azmanov, Atkinson, Black, Baynam, Dreyer, Hayeems, Marshall, Costain, Wessels, Baptista, Drummond, Leffler, Field and Gecz.
Figures




References
-
- Brook J. D., Mccurrach M. E., Harley H. G., Buckler A. J., Church D., Aburatani H., et al. (1992). Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 69:385. 10.1016/0092-8674(92)90418-c - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

