Indoleamine-2,3-Dioxygenase Mediates Emotional Deficits by the Kynurenine/Tryptophan Pathway in the Ethanol Addiction/Withdrawal Mouse Model
- PMID: 32116558
- PMCID: PMC7026684
- DOI: 10.3389/fncel.2020.00011
Indoleamine-2,3-Dioxygenase Mediates Emotional Deficits by the Kynurenine/Tryptophan Pathway in the Ethanol Addiction/Withdrawal Mouse Model
Abstract
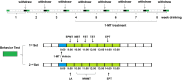
Objective: Our study was designed to investigate whether the indoleamine-2,3-dioxygenase (IDO)-mediated kynurenine/tryptophan (KYN/TRP) pathway participates in the development of emotional deficits from ethanol addiction/withdrawal mice.
Methods: The expression of proinflammatory factors, including tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), was tested by enzyme-linked immunosorbent assay (ELISA). The IDO levels in the hippocampus, cerebral cortex, and amygdala were measured by polymerase chain reaction (PCR) and western blot, and the neurotransmitters were tested by high performance liquid chromatography (HPLC). Emotional deficits of mice were evaluated by behavioral tests.
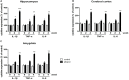
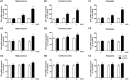
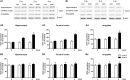
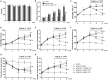
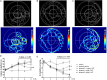
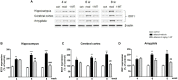
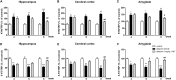
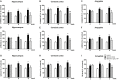
Results: Expression levels of inflammatory factors (TNF-α, IL-1β, and IL-6) were increased in mice after 4 weeks of alcohol exposure. As for indoleamine 2,3-dioxygenase (IDO) expression, only the subtype IDO1 was found to increase at both mRNA level and protein level in all the tested brain regions of ethanol addiction/withdrawal mice. In behavioral tests, mice exposed to alcohol showed gradually declined memory function accompanied by anxiety-like and depressive-like behaviors. Meanwhile, increased expression of KYN, decreased expression of 5-HT, and abnormal expression of 3-HK and KA were found in the hippocampus, cerebral cortex, and amygdala of ethanol addiction/withdrawal mice. Interestingly, the IDO1 inhibitor, 1-methyl-L-tryptophan (1-MT), reversed all above alterations induced by ethanol in mice.
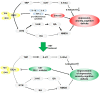
Conclusion: Our results suggested that the TRP/KYN pathway, medicated by IDO1, in the hippocampus, cerebral cortex, and amygdala, plays an important role in the development of emotional deficits caused by ethanol addiction and withdrawal.
Keywords: 3-dioxygenase; anxiety; depression; ethanol addiction; indoleamine 2; memory.
Copyright © 2020 Jiang, Lin, Xu, Chen, Yan, Chen and Yu.
Figures
Similar articles
-
Role of the indoleamine-2,3-dioxygenase/kynurenine pathway of tryptophan metabolism in behavioral alterations in a hepatic encephalopathy rat model.J Neuroinflammation. 2018 Jan 4;15(1):3. doi: 10.1186/s12974-017-1037-9. J Neuroinflammation. 2018. PMID: 29301550 Free PMC article.
-
Cang-Ai Volatile Oil Ameliorates Depressive Behavior Induced by Chronic Stress Through IDO-Mediated Tryptophan Degradation Pathway.Front Psychiatry. 2021 Dec 15;12:791991. doi: 10.3389/fpsyt.2021.791991. eCollection 2021. Front Psychiatry. 2021. PMID: 34975590 Free PMC article.
-
Inhibition of Indoleamine 2,3-Dioxygenase Exerts Antidepressant-like Effects through Distinct Pathways in Prelimbic and Infralimbic Cortices in Rats under Intracerebroventricular Injection with Streptozotocin.Int J Mol Sci. 2024 Jul 8;25(13):7496. doi: 10.3390/ijms25137496. Int J Mol Sci. 2024. PMID: 39000602 Free PMC article.
-
Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway.Redox Rep. 1999;4(5):199-220. doi: 10.1179/135100099101534927. Redox Rep. 1999. PMID: 10731095 Review.
-
The new '5-HT' hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression.Prog Neuropsychopharmacol Biol Psychiatry. 2011 Apr 29;35(3):702-21. doi: 10.1016/j.pnpbp.2010.12.017. Epub 2010 Dec 23. Prog Neuropsychopharmacol Biol Psychiatry. 2011. PMID: 21185346 Review.
Cited by
-
Implications of Kynurenine Pathway Metabolism for the Immune System, Hypothalamic-Pituitary-Adrenal Axis, and Neurotransmission in Alcohol Use Disorder.Int J Mol Sci. 2024 Apr 29;25(9):4845. doi: 10.3390/ijms25094845. Int J Mol Sci. 2024. PMID: 38732064 Free PMC article. Review.
-
Combining Metabolomics and Interpretable Machine Learning to Reveal Plasma Metabolic Profiling and Biological Correlates of Alcohol-Dependent Inpatients: What About Tryptophan Metabolism Regulation?Front Mol Biosci. 2021 Nov 8;8:760669. doi: 10.3389/fmolb.2021.760669. eCollection 2021. Front Mol Biosci. 2021. PMID: 34859050 Free PMC article.
-
Genome-wide interaction association analysis identifies interactive effects of childhood maltreatment and kynurenine pathway on depression.Nat Commun. 2025 Feb 18;16(1):1748. doi: 10.1038/s41467-025-57066-4. Nat Commun. 2025. PMID: 39966400 Free PMC article.
-
Effects of E2 on the IDO1-mediated metabolic KYN pathway in OVX female mice.J Cell Mol Med. 2024 Oct;28(20):e70179. doi: 10.1111/jcmm.70179. J Cell Mol Med. 2024. PMID: 39467780 Free PMC article.
-
Irisin attenuates ethanol-induced behavioral deficits in mice through activation of Nrf2 and inhibition of NF-κB pathways.Metab Brain Dis. 2023 Jun;38(5):1643-1656. doi: 10.1007/s11011-023-01202-w. Epub 2023 Mar 22. Metab Brain Dis. 2023. PMID: 36947333
References
-
- André C., O’Connor J. C., Kelley K. W., Lestage J., Dantzer R., Castanon N. (2008). Spatio-temporal differences in the profile of murine brain expression of proinflammatory cytokines and indoleamine 2,3-dioxygenase in response to peripheral lipopolysaccharide administration. J. Neuroimmunol. 200 90–99. 10.1016/j.jneuroim.2008.06.011 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

