Effects of Limited and Extended Pavlovian Training on Devaluation Sensitivity of Sign- and Goal-Tracking Rats
- PMID: 32116587
- PMCID: PMC7010919
- DOI: 10.3389/fnbeh.2020.00003
Effects of Limited and Extended Pavlovian Training on Devaluation Sensitivity of Sign- and Goal-Tracking Rats
Abstract
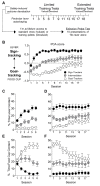
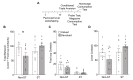
Individual differences in Pavlovian approach predict differences in devaluation sensitivity. Recent studies indicate goal-tracking (GT) rats are sensitive to outcome devaluation while sign-tracking (ST) rats are not. With extended training in Pavlovian lever autoshaping (PLA), GT rats display more lever-directed behavior, typical of ST rats, suggesting they may become insensitive to devaluation with more Pavlovian training experience. Here, we use a within-subject satiety-induced outcome devaluation procedure to test devaluation sensitivity after limited and extended PLA training in GT and ST rats. We trained rats in PLA to determine GT and ST groups. Then, we sated rats on either the training pellets (devalued condition) or homecage chow (valued condition) prior to brief non-reinforced test sessions after limited (sessions 5/6) and extended (sessions 17/18) PLA training. GT rats decreased conditioned responding under devalued relative to valued conditions after both limited and extended training, demonstrating they are sensitive to satiety devaluation regardless of the amount of PLA training. While ST rats were insensitive to satiety devaluation after limited training, their lever directed behavior became devaluation sensitive after extended training. To determine whether sign-tracking rats also displayed sensitivity to illness-induced outcome devaluation after extended training, we trained a separate cohort of rats in extended PLA and devalued the outcome with lithium chloride injections after pellet consumption in the homecage. ST rats failed to decrease conditioned responding after illness-induced outcome devaluation, while Non-ST rats (GT and intermediates) were sensitive to illness-induced outcome devaluation after extended training. Together, our results confirm devaluation sensitivity is stable in GT rats across training and devaluation approaches. Extended training unmasks devaluation sensitivity in ST rats after satiety, but not illness-induced devaluation, suggesting ST rats respond appropriately by decreasing responding to cues during state-dependent but not inference-based devaluation. The differences in behavioral flexibility across tracking groups and devaluation paradigms have translational relevance for the understanding state- vs. inference-based reward devaluation as it pertains to drug addiction vulnerability.
Keywords: behavioral flexibility; goal-tracking; outcome devaluation; pavlovian incentive learning; reward; sign-tracking.
Copyright © 2020 Keefer, Bacharach, Kochli, Chabot and Calu.
Figures


References
-
- Adams C. D. (1982). Variations in the sensitivity of instrumental responding to reinforcer devaluation. Q. J. Exp. Psychol. 34, 77–98. 10.1080/14640748208400878 - DOI
-
- Boakes R. A. (1977). “Performance on learning to associate a stimulus with positive reinforcement,” in Operant-Pavlovian Interactions, ed. Hurwitz I. H. D. H. M. B. (Hillsdale, NJ: Erlbaum; ), 67–101.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

