MicroRNA-96 Promotes Vascular Repair in Oxygen-Induced Retinopathy-A Novel Uncovered Vasoprotective Function
- PMID: 32116694
- PMCID: PMC7008172
- DOI: 10.3389/fphar.2020.00013
MicroRNA-96 Promotes Vascular Repair in Oxygen-Induced Retinopathy-A Novel Uncovered Vasoprotective Function
Abstract
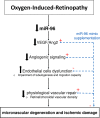
Background and aims: Vascular degeneration is a hallmark in the pathogenesis of oxygen-induced retinopathy (OIR). Dysregulation of microRNAs (miRNAs), key regulators of genes expressions, has been implicated in the regulation of ocular angiogenesis. However, miRNAs specific functions in impaired vascular development during OIR are poorly understood. Herein, we identified miR-96 as one of the most highly expressed miRNAs in the retina and choroid during vascular development and investigated the potential role of miR-96 on microvascular degeneration in a rat OIR model.
Methods and results: Next generation sequencing (NGS) and qRT-PCR analysis showed that miR-96 maintain high levels of expression during ocular vascular development. Nevertheless, miR-96 was significantly downregulated in the retina and choroid of OIR rats (80% O2 from P5 to P10) during the phase of microvascular degeneration. Similarly, human retinal microvascular endothelial cells (HRMEC) subjected to hyperoxia (80% O2) showed a significant downregulation of miR-96 evaluated by qPCR. Interestingly, HRMEC supplemented with miR-96 regulated positively the expression of several key angiogenic factors including VEGF and ANG-2. To explore the angiogenic activity of miR-96 on HRMEC, we performed a gain/loss of function study. In a similar way to hyperoxia exposure, we observed a robust angiogenic impairment (tubulogenesis and migration) on HRMEC transfected with an antagomiR-96. Conversely, overexpression of miR-96 stimulated the angiogenic activity of HRMEC and protected against hyperoxia-induced endothelial dysfunction. Finally, we evaluated the potential vasoprotective function of miR-96 in OIR animals. Rat pups intravitreally supplemented with miR-96 mimic (1 mg/kg) displayed a significant preservation of retinal/choroidal microvessels at P10 compared to controls. This result was consistent with the maintenance of physiologic levels of VEGF and ANG-2 in the OIR retina.
Conclusion: This study demonstrates that miR-96 regulates the expression of angiogenic factors (VEGF/ANG-2) associated to the maintenance of retinal and choroidal microvasculature during physiological and pathological conditions. Intravitreal supplementation of miR-96 mimic could constitute a novel therapeutic strategy to improve vascular repair in OIR and other ischemic retinopathies.
Keywords: endothelial dysfunction; micro-RNA (miRNA); oxygen-induced retinopathy (OIR); vascular degeneration; vascular repair and angiogenesis.
Copyright © 2020 Desjarlais, Wirth, Rivera, Lahaie, Dabouz, Omri, Ruknudin, Borras and Chemtob.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

